 https://orcid.org/0000-0002-0785-6237
https://orcid.org/0000-0002-0785-6237Kozminski University https://orcid.org/0000-0002-0785-6237
https://orcid.org/0000-0002-0785-6237
Abstract:
Cooperation between the pharmaceutical industry and medical doctors is both necessary and inevitable. As part of the medical community, medical students are likely to have developed specific views as to its benefits and risks. The article is part of a series of articles presenting selected results of nine focus group interviews from three cities (Warsaw, Kraków, and Gdańsk). The study used social constructivism as a theoretical framework, and thematic analysis was performed. In their group discussions, the students drew links between cooperation’s perceived benefits and risks. Despite the associated risks, most medical students perceive benefits obtained from the pharmaceutical industry as attractive. Better education about social, psychological issues (manipulation techniques) and the conflict of interest is needed in medical schools.
Keywords:
sociology of medicine, social psychology, medical education, pharmaceutical industry
Abstrakt:
Współpraca przemysłu farmaceutycznego z lekarzami jest konieczna i nieunikniona. Studenci medycyny, jako część społeczności medycznej, prawdopodobnie rozwinęli specyficzne poglądy na temat korzyści i zagrożeń z nią związanych. Artykuł jest częścią cyklu przedstawiającego wybrane wyniki dziewięciu zogniskowanych wywiadów grupowych z trzech miast – Warszawy, Krakowa i Gdańska. W badaniu wykorzystano konstruktywizm społeczny jako ramę teoretyczną i przeprowadzono analizę tematyczną. W dyskusjach grupowych studenci stworzyli powiązania między postrzeganymi korzyściami i zagrożeniami we współpracy lekarzy z przemysłem. Choć przyszli lekarze dostrzegali wiele ryzyk, to jednak korzyści uzyskiwane od przedstawicieli przemysłu wydawały im się bardzo atrakcyjne. Badanie wskazuje, że na uczelniach medycznych potrzebna jest lepsza edukacja w zakresie zagadnień społecznych, psychologicznych (sposobów manipulacji) oraz konfliktu interesów.
Słowa kluczowe:
socjologia medycyny, psychologia społeczna, edukacja medyczna, przemysł farmaceutyczny
The main aim of this article is to identify and describe medical students’ viewpoints regarding cooperation between medical doctors (henceforth referred to as doctors for brevity) and the pharmaceutical industry. The article seeks to systematize knowledge related to this issue and further enrich the knowledge base with empirical research results. The industry uses a range of manipulation techniques to influence doctors’ decisions and prescribing practices, and makes effective use of Robert Cialdini’s (2001) principles of social influence: reciprocity, commitment and consistency, social proof, liking, authority, and scarcity. Marketing aimed at doctors utilizes subtle forms of manipulation, such as the use of key leaders of opinion, disease mongering, and the provision of repeated medical education events (Leonardo Alves, Lexchin, Mintzes, 2019; Makowska, Nowakowski, 2020).
Making a correct decision as to which drug to prescribe to a patient is complex: among other things, a doctor must take into account the goals of the patient’s therapy, its socioeconomic implications, and the advantages and disadvantages of the drug compared to other drugs on the market. To do all this, the doctor must have a knowledge of the range of marketed products. Cooperation with the industry can lead doctors to have conflicts of interest, with ethical and professional behaviors possibly clashing with financial self-interests (Dana, Loewenstein, 2003). Such conflicts of interest can lead to suboptimal prescription of medicaments and even over-prescribing. Real or perceived conflicts of interest undermine patients’ trust in doctors and the pharmaceutical industry, and Anthony Giddens (2006) has noted that we need to trust authorities if we want to confront the risks around us.
Because cooperation between doctors and pharmaceutical companies is common (Campbell et al., 2007; Lieb, Brandtönies, 2010), it is often observed by medical students. The impact of such observations can be better understood with the help of the theory of reference group behavior. Here, Robert Merton has stated that people compare themselves to other people who have a social position that they would like to achieve (Merton, Kitt, 1950). In the present case, medical students compare themselves with practicing doctors, who are their role models. But “role models may not be a dependable way to impart professional values, attitudes, and behaviours” (Paice, Heard, Moss, 2002: 707). Observing practicing doctors interacting in various ways with the pharmaceutical industry may lead students to accept such behavior as the norm. This is supported by Cialdini’s social proof principle: “we determine what is correct by finding out what other people think is correct” (Cialdini, 2001: 100).
A student’s approach to the pharmaceutical industry may also be influenced by their institution’s policy concerning the industry. Research has shown that medical students and residents studying in institutions with restrictive policies regarding gifts and contacts with pharmaceutical sales representatives (PSRs) show a stricter approach to business in their future careers (McCormick et al., 2001; King et al., 2013).
In 2007, in the US, the American Medical Student Association (AMSA) started to assess medical schools’ conflict of interest policies. Initially, the AMSA simply checked for the presence of such policies, but since 2008, in collaboration with the Pew Prescription Project, it has used an elaborate scorecard including 11 policy domains, among which were gifts and meals from the pharmaceutical industry, funded educational programs, and scholarships (Carlat et al., 2016). Also, research on medical schools’ policies has been conducted in Australia (Mason, Tattersall, 2011), Canada (Shnier et al., 2013), France (Scheffer et al., 2017), Germany (Grabitz et al., 2020), and Belgium (Bechoux et al., 2021). Unfortunately, all of this research has concluded that there is little to protect medical students from the influence of the industry. More encouragingly though, media publicity relating to the rankings obtained by US and French medical schools with respect to the adequacy of their policies has resulted in changes for the better (Bechoux et al., 2021).
It is worth noting that Polish medical schools still lack policies regarding the relationships at issue. Medical studies last 6 years. Issues surrounding cooperation with the pharmaceutical industry are discussed in a perfunctory manner, and there is no well-thought-out program addressing problematic issues concerning this cooperation. It should also be noted that in Poland, PSRs’ visits to doctors’ offices are common (Makowska, 2017), and this is probably one reason why the Polish pharmaceutical market has been growing for many years; in 2022, the value of the market was 45,4 billion PLN (IQVIA, 2023).
The present article aims to answer the following research question: ‘What links do students draw between perceived benefits and risks of the doctor–pharmaceutical industry cooperation?’ The article stems from a broader study investigating pharmaceutical companies’ impact on the socialization of medical students in Poland. Further details can be found in previously published articles relating to this research project (Makowska, 2022; Makowska, Kaczmarek, Rodzinka, 2022). It should be noted that none of these previous articles had covered the topic of perceived risks and benefits of cooperation with the pharmaceutical industry, although these articles included brief discussions of students’ opinions about PSR gift-giving (Makowska, 2022; Makowska, Kaczmarek, Rodzinka, 2022) and loss of patient trust (Makowska, Kaczmarek, Rodzinka, 2022).
Focus group interviews (FGIs) were used to collect data. Nine focus groups consisting of medical students were used, with an average of 10 students per group (N = 92 students overall; 52 females and 40 males). A condition for participation was that students were in at least their second year of studying medicine (there were 7 second years, 41 third years, 20 fourth years, 11 fifth years, and 13 sixth years). The research was conducted in three cities – Gdańsk, Warsaw, and Kraków – in November 2019.
All students were recruited by an external recruitment company in one of two ways. First, by approaching them via relevant Internet groups. Second, by a recruiter visiting the university precincts in person and asking passing students whether they were studying medicine, and, if so, whether they were interested in participating. Irrespective of the method of recruitment, the company informed students about the project’s aims, the source of its funding, and the terms of participation. Focus group sessions took place off campus on rented premises specially designed for such sessions. Students were asked to attend these premises within a designated timeframe if they were interested in participating in the research. Students attending a FGI received 150 PLN (33 EUR) and were allowed to leave FGIs at any time. The study was not reviewed by an Ethics Committee as Polish regulations did not require ethical approval at the time the study was conducted, but reasonable measures were taken to ensure students’ confidentiality. Transcription was done by the same company that was responsible for recruitment. Video recordings were only accessible to the researcher and one individual responsible for reviewing transcriptions derived from audio recordings; during this process, the students’ first names were changed.
The scenario for all FGIs was prepared in advance and consisted of three modules (a familiarization part – 10 minutes, a part dealing with being socialized to the role of a doctor – 50 minutes, and a part covering students’ relationships with the pharmaceutical industry – 60 minutes). This sequence was the same for each group. The researcher, who was also a moderator, sought to gain the trust of students slowly, by starting with topics that were easy for them to discuss during the first hour and only then moving on to more detailed and sensitive topics which formed the heart of the research. Each focus group session lasted approximately two hours. The focus group scenario and transcripts are available publicly at: https://doi.org/10.18150/UFEA7T, V1.
Because focus group discussions encourage the disclosure of different, often contradictory, views, the FGI technique was an effective way of obtaining information about students’ perceptions of the risks and benefits of cooperating with the pharmaceutical industry. The FGI technique has noteworthy benefits in allowing an extensive amount of information to be obtained from numerous individuals in a relatively brief timeframe. However, in the present study, the technique also had a drawback in that it was not possible to assemble groups of participants who were unacquainted with each other, because students were studying at the same university. While this might have mitigated the likelihood of insincerity, apprehensiveness associated with being negatively judged by one’s acquaintances might have restricted students from expressing certain points of view. Additionally, the fact that FGI interviews are recorded on video may have impeded some individuals from voicing their opinions.
The study adopted a social constructivist framework. Here, Peter L. Berger and Thomas Luckman (1966) claimed that knowledge and the way we perceive reality is socially constructed. They underlined the role of socialization, arguing that humans are not born as members of society, but become members of society through socialization. Sharon Derry (1999) notes that social constructivists emphasize the importance of culture and context in understanding societal functioning, and assumes that the construction of knowledge is based on this understanding. Every conversation between two people is a chance to acquire new knowledge and to discuss meanings. Social constructivists hold the view that reality is inseparable from the contexts and meanings in which it is situated.
The social constructivist approach is well suited to focus group analysis as the latter focuses on how groups construct certain meanings in the course of discussion, and how groups reach or fail to reach a consensus on certain issues (Stewart, Shamdasani, Rook, 2007). Scholars using a constructivist framework reject the idea that there is an objective “truth”, but, rather, they argue that there may be multiple valid versions of it (Zboroń, 2009).
Peter L. Berger and Thomas Luckman (1966: 116) state that “To understand the state of the socially constructed universe at any given time, or its change over time, one must understand the social organization that permits the definers to do their defining.” Thus, students existing within a medical culture may perceive and understand cooperation with the pharmaceutical industry differently than other people, and see different benefits and risks. Hence, ascertaining students’ opinions on this issue is very important. The material collected presently was subjected to thematic analysis in accordance with the six steps outlined in the guidelines of Virginia Braun and Vicotria Clarke (2006). In this analysis, Robert Cialdini’s (2001) principles of influence were used following the steps outlined in previous publications (e.g. Makowska, 2010; Sah, Fugh-Berman, 2013).
Themes such as the provision of gifts/meals, educational materials, drug samples, professional growth opportunities, and the relations with PSRs were recognized as benefits, and various risk subthemes were connected with each benefit. This is illustrated in Figure 1.
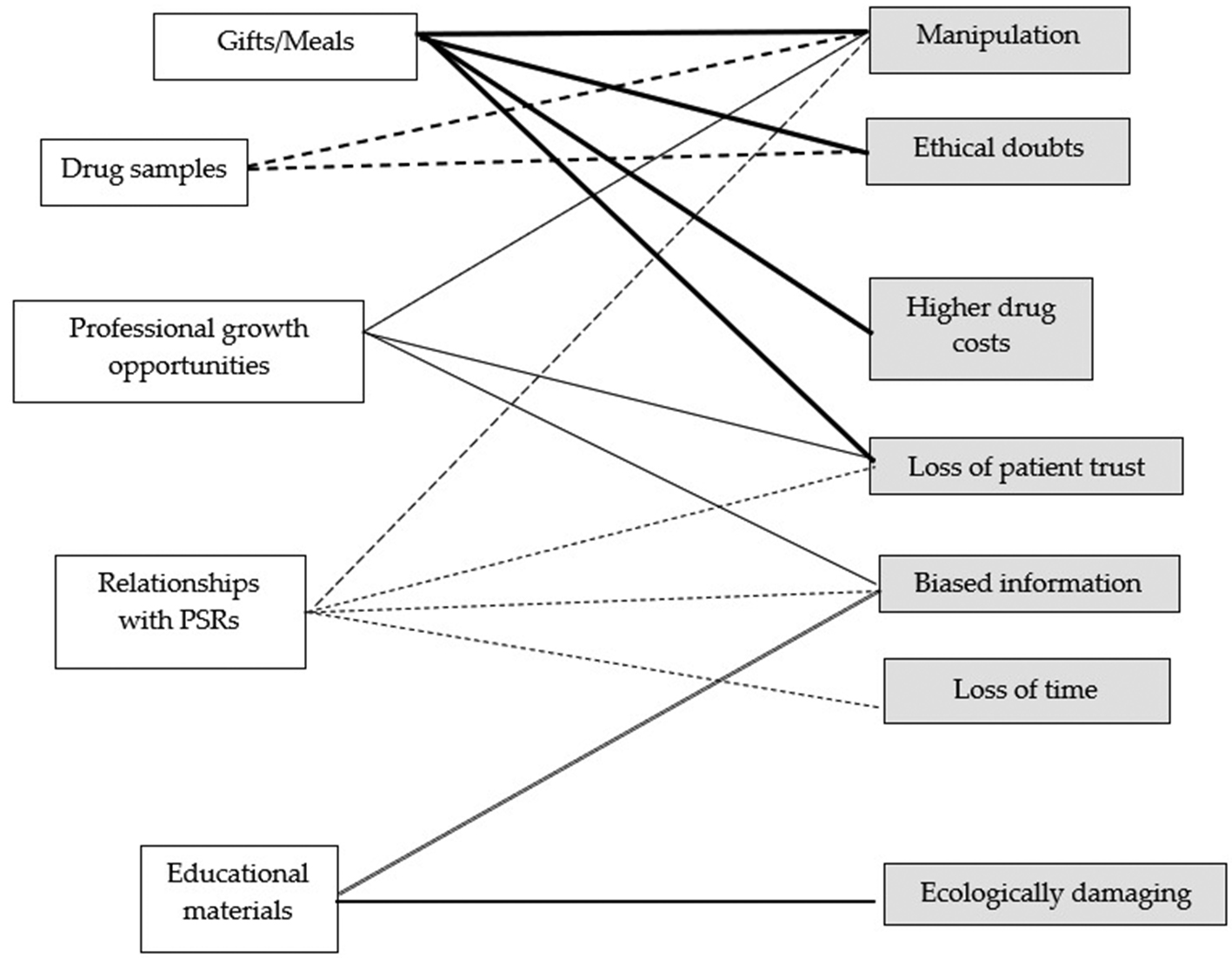
Figure 1. Results of thematic analysis
Source: own research.
The group discussions uncovered various ideas about the benefits enjoyed by doctors through their cooperation with the pharmaceutical industry. Often, when one student mentioned a benefit, other students responded, pointing to risks associated with the benefit.
In each of the focus groups, students mentioned the provision of gifts as an advantage of cooperating with the pharmaceutical industry (these gifts were both educational and non-educational, e.g. reprints, books, mugs, notebooks, calendars, bags, and USB flash drives). They also mentioned companies buying reagents for science clubs. The acceptance of gifts was often excused by the poor state of the Polish health system, and gifts were sometimes seen as constituting help for underfunded institutions and their staff. Students also noticed an imbalance in the nature of these gifts, more expensive gifts being given to doctors than to students or nurses. There was also an appreciation of the meals that PSRs gave to medical societies, both small snacks and expensive lunches being mentioned. Students believed that patients also benefited from the companies’ gifts, some items being passed on to patients; here, stickers, cards providing medical information, and glucose meters were mentioned.
Well, I would like to point out that these gifts are important anyway, because hospitals are so poorly financed […]. (M – male, 6th year)
[…] if the PSRs came with gloves, I think everyone would be happy. Because I myself remember a situation when I was supposed to inject a patient in my first year and I got plastic gloves [for handling food – author] from the store (group laughter). No, it was not funny for me. (F – female, 5th year; Warsaw, GP 9)
The intentions of companies that give gifts or meals to medical staff were often discussed. Many students noted that such gift-giving is not altruistic, rather, in six of the groups it was mentioned that such actions were likely to be used as a means of forcing doctors to reciprocate in some way. Here, while the term ‘reciprocity principle’ was only occasionally used (see the example below), students often described the mechanism as being in action without naming it.
It works by activating very basic instincts, such as the reciprocity principle […]. I was supposed to prescribe this substance to my patients, and this PSR is so nice, so I prescribed it. In any case, the patient could change it at the pharmacy. (M, 3rd year; Warsaw, GP 9)
Another explanation of gift-giving was to keep a company/product name in a doctor’s mind by means of a logo: having a logo in front of their eyes when they are writing prescriptions. But the idea that taking gifts with different logos from many companies could be a possible defense against this practice was discussed by students. Also, during discussions about manipulation, many students expressed the opinion that doctors are rational people and that such mechanisms should not be able to influence them.
Some students had ethical concerns about taking gifts. These concerns were most often about gifts rather than meals. The term ‘conflict of interest’ was used by students in only one group’s discussions (an example is provided below). In most groups, only the general idea that accepting gifts raised ethical questions was discussed, and the notion of conflicts of interest was described rather than explicitly referred to.
Such a disadvantage could actually be called a conflict of interest, because, when they provide things, they can encourage the use of a specific drug, and the doctor decides for the patient because this is their function. At this point, there may be a [implicit – author] suggestion that there is some debt to be paid […]. (M, 4th year; Gdańsk, GP 1)
Students also noted that giving gifts/meals to the medical community could have a negative impact on the cost of drugs for patients because someone has to pay for these benefits. Some students also feared that marketing may also cause some doctors to prescribe unnecessary or expensive drugs. In one of the groups there was also a thread concerning doctors recommending placebos to patients, and also prescribing drugs when it would be better to recommend a lifestyle change. One student talking about this said:
[…] If the doctor receives any benefit from prescribing a drug […] then they might prescribe it to patients who do not need the drug […]. (F, 4th year; Warsaw, GP 7)
In all groups, it was also noted that patients might lose trust in doctors due to them seeing pharmaceutical company gifts in doctors’ offices, and patients receiving treatment recommendations on sheets of paper containing medicament logos. Many students thought that it would be difficult for patients to perceive the benefits of doctors’ cooperation with pharmaceutical companies, not least because the Polish media prefers to show the bad side of cooperation. Part of a discussion about this problem is presented below:
Patient trust can be lost. (M, 6th year)
Yes, in their eyes we will be corruptible. (F1, 3rd year)
[…]
This is based on [the suspicion – author] that this one [PSR – author] bought him a trip and the other one [PSR – author] something else. (M, 6th year)
Moderator: I walk into the office, and what do I see?
Tissues, pens. (F2, 3rd year)
[…]
Only logos. (F1, 3rd year; Kraków, GP5)
In eight focus groups, students noted that pharmaceutical companies provide doctors professional growth opportunities, helping them to collect educational credits which are required by the Polish legislation (Official Journal of Laws of 2022, item 464). The industry sponsors conferences, courses, and training events. Part of such a conversation is recounted below.
[…] There are courses and conferences [sponsored by pharmaceutical companies – author], and a doctor who leaves his workplace for a conference collects some credits for it […]. There are lectures by other doctors at such conferences. (F1, 3rd year)
And during the breaks, in the corridors there are a lot of different stands [of pharmaceutical companies – author]. (F2, 3rd year; Kraków, GP5)
Additionally, students talked about the fact that companies also organize their own meetings for doctors, which have mainly marketing goals. Events of this type are in attractive places (e.g. expensive hotels), and lectures are conducted by doctors who talk about a given disease, for which a company – the sponsor – produces a drug. In many groups, students praised pharmaceutical companies for providing knowledge in a very simple way, and it was pointed out that doctors can obtain knowledge of drugs by participating in such meetings, and that this enables them to become aware of the availability of a wider range pharmaceuticals than would otherwise be the case. The students also pointed out that pharmaceutical companies enable some doctors to participate in clinical trials. The professional growth opportunities provided by the pharmaceutical industry benefit patients, because they often receive better treatment owing to doctors’ enhanced knowledge. One student described the meetings at issue in the following way:
[…] there were, for example, lectures by a gentleman from some concern, who promoted a drug for… it was probably obesity. Later, the diabetologist discussed something about diabetes, and at the end said that [there are – author] new drugs, such and such, produced by the sponsoring company [of the meeting – author] […]. Each lecture was summarized – who, how, and which sponsor. (M, 3rd year; Warsaw, GP 7)
However, as in the case of gifts and meals, there were skeptical students saying that the pharmaceutical industry’s financing of professional growth opportunities constitutes a form of manipulation. An example of this type of statement is as follows:
[…] in a first year lecture, a professor said that […] pharmaceutical companies do not demand anything directly, they only send them [doctors – author] on courses, finance them, finance equipment, and then, it’s not that you sign a contract, only that the doctor simply feels obligated in some way. (M, 3rd year; Kraków, GP 5)
The respondents also pointed out that doctors’ attendance at such external conferences may result in patients having the unflattering opinion that these conferences are, in fact, holidays. And it was thought that this could lead to a loss of confidence among patients. Here is one student’s view of the issue:
[…] there is an unflattering public opinion that, for example, doctors go on vacation with the money provided by large pharmaceutical companies; that they sponsor them [doctors – author] to do such pleasant things. (M, 3rd year; Kraków, GP 4)
Students noted that information from the pharmaceutical industry needs to be checked, because it can be selective, sometimes being chosen to cast the performance of a company’s drug in the best possible light. Disadvantages are often overlooked, the main focus being on a drug’s advantages. They expressed the opinion that obtaining information from pharmaceutical companies may cause doctors to become uncritical, or even to become addicted to building their knowledge using this information. Students also did not trust drug manufacturers’ research. One student expressed this concern as follows:
These studies on effectiveness are also carried out by the company, they are methodologically biased, you know they are… […] (M, 3rd year; Warsaw, GP 9)
The pharmaceutical industry also provides doctors with a variety of educational materials – from reprints and textbooks to simple materials that are also intended to help patients. As the following discussion illustrates, some students considered this to be one of the benefits of cooperating with the industry:
[…] For example, companies give handouts for patients on how to measure insulin. This is cool, because normally a doctor would have to write it down, but now they already have it [the information – author]. (M1, 3rd year)
I saw the diabetic diet flyers, all sorts of things, very useful. (M2, 4th year; Warsaw, GP 9)
As was the case with professional growth opportunities, students made the accusation that the educational materials created by companies sometimes contained inappropriate information. An example of such a statement is as follows:
I mean, often the leaflet they give says that, for example, [a drug – author] is 70% more effective than the standard version of the drug. It’s just that you don’t really know, you would have to check some scientific research on the Internet, just… is it really so? Because it is not known what it is being compared to, in fact, it maybe to the same drug but in a smaller dose […]. (M, 5th year; Warsaw, GP 7)
In two of the groups, students pointed out that educational materials for doctors or patients often end up in the garbage. Some members of this new generation of students, who already had pro-ecological attitudes, felt that such a type of promotion should be abandoned, as the following statement shows:
[…] something that annoys me a lot, […] is unecological approach to these leaflets. There is such a large number of them, and it’s just… they end up in the trash bin anyway, and both sides realize this, I think. (F, 3rd year; Kraków, GP 6)
Also, students perceived relationships with PSRs as beneficial. PSRs constitute an easy way of acquiring information, and it was emphasized, for example, that it is usual for them to supply doctors with information about things such as drug offerings, medicine prices, reimbursement, new indications, new forms of administration, composition modifications, name changes, and drug recalls. An example of a discussion of this issue is provided below.
[…] The possibility of getting to know [drugs – author], because not all doctors have time. They [PSRs] can keep them on track, and therefore they can be up-to-date […] (F1, 4th year)
[…] obtaining information about the price of a drug, because, for example, it is important for some patients as to whether they have a more expensive and cheaper drug to choose from. (F2, 5th year; Gdańsk, GP1)
The students also pointed out that some doctors may like some PSRs who visit them, and enjoy spending time with them. PSRs are often rather young, pleasant people, who are well dressed, talkative, open-minded, and physically attractive. Some students believed that a doctor can also establish valuable contacts through PSRs – for example, at the meetings they organize. Part of such a discussion is given below.
They [PSRs – author] want to gain the trust of another human being, […], to please someone a little bit… (F1, 6th year)
Yes, they always glorify: ‘Oh Mr. Doctor is so…’ (M1, 6th year)
Gain someone’s attention. (F2, 6th year)
Forever happy, easy going […]. (M2, 4th year; Gdańsk, GP2)
Risks of relationships with PSRs – manipulation
There were also voices pointing to the risk of relationships between doctors and PSRs. In some group discussions, it was pointed out that PSRs might use friendly relationship as a way of influencing doctors’ decisions. An example of such a statement is given below.
[…] we can even make friends with them [PSRs – author] somehow, and that somehow obliges [us – author] […]. (F, 4th year; Warsaw, GP 7).
Risks of relationships with PSRs – loss of patient trust
Students also pointed out that seeing PSRs in doctors’ offices might result in patients losing trust. Here is an example of such a statement:
I have the impression that, for a patient, when he sees a PSR in the doctor’s office, it is enough to create his own [unfavorable – author] opinion, because he is unaware of many mechanisms as to why this cooperation exists and what the benefits are. That it could be for his own good too, right? And not necessarily that the doctor just gets rich […]. (F, 5th year; Gdańsk, GP 2)
Risks of relationships with PSRs – the provision of biased information
PSR visits to doctors’ offices were not perceived by most students as providing a reliable source of information. In some discussions, there was also a thread involving the idea that PSRs do not provide information about side effects of drugs unless doctors ask about this themselves. Focus group participants often stressed that what PSRs say should be verified, as shown in the following example:
We know that it is gigantic marketing, and that they are mainly salespeople, not specialists in a given field. However, it seems to me that you need to get as much as possible for yourself from such meetings, and read about the active substance later; maybe look for an article on the topic, find out, educate yourself […]. (F, 3rd year; Kraków, GP 4)
Risks of relationships with PSRs – loss of time
In all of the groups, attention was drawn to the idea that PSRs take up time that doctors should devote to patients: that PSRs distract doctors from their work. Many students witnessed PSRs queuing in lines of patients. Some noticed that this was a problem not only for the doctor, but also for the patients, who had to wait longer for an appointment. In most groups, students thought that PSRs are sometimes pushy, insistent, artificially nice, and self-interested, and that some doctors often do not know how to get rid of them:
It is irritating that even if there is a long queue, they come in when they know there are patients. For example, they don’t come at the end, they just come and walk in brutally between patients. It irritates me a bit, to be honest […]. (M, 3rd year; Kraków, GP 5)
Sometimes, pharmaceutical companies make large drug donations to medical institutions – and students saw this as an important element of cooperation between the medical community and drug manufacturers. They also mentioned that doctors receive drug samples from PSRs. They can use these in various ways: keep them for themselves (then they perform a marketing function, just like a gift), hand them over to the institution in which they work, or give them to a patient in need. Students often saw benefits for patients in the provision of samples, as in the following statement:
Also, when a doctor gets some free samples of drugs, the patient can use the drug before buying a full package: they can check whether it is worth [buying the drug – author]. (F, 3rd year; Kraków, GP 4)
Risks of providing drug samples – manipulation
Some students said that the provision of drug samples invokes the reciprocity rule, as these are perceived as gifts. In two of the groups, it was noted that drug samples may not necessarily be as beneficial to patients as they seem at first glance: sometimes it might be the case that a patient unnecessarily continues therapy with a drug which was initially given to them as a sample, and that this ultimately brings an unwarranted profit to the company. One student described the situation like this:
[…] just this patient, if he gets this drug for free, maybe later he will thoughtlessly continue using the drug […]. (M, 3rd year; Gdańsk, GP2)
Risks of providing drug samples –ethical doubts
Accepting drug samples was viewed as ethically questionable. Here, starting new therapy with free samples raised particular doubts (described above), as did situations where doctors use free drug samples for their own purposes. Part of a discussion where students shared their doubts about the latter issue is shown below:
[…] How should it [the drug sample – author] be used? (M1, 4th year)
Sell it? (K1, 3rd year)
For free for patients, or for ourselves? I do not know who is the target? (K2, 4th year)
[…]
I think a lot depends on what drug it is – this is the first thing, and the second thing is if anyone wants it. Actually, I don’t know what other use of this drug there could be other than for a doctor’s own purposes. […], it doesn’t actually turn out to be a bribe. (M1, 4th year; Gdańsk, GP1)
Cooperation between the medical community and the pharmaceutical industry is both inevitable and desirable, but it also carries many risks. In group discussions, medical students saw both positive and negative sides to cooperation.
The students saw the Polish health service’s poor financial situation as being largely responsible for doctors’ cooperation with the pharmaceutical industry, and as being behind their acceptance of gifts. In all groups, there was a discussion about accepting a sandwich from PSRs and, even after discussing benefits and risks, the vast majority in all groups said that they would have no problem accepting a sandwich since they considered themselves to be needy students. These types of finding are consistent with German research where almost half of the medical students used their poor financial situation as a justification for accepting gifts (Lieb, Koch, 2013).
The present students shared the opinion that doctors’ behaviors are rational and they thought that accepting gifts and meals did not influence doctors to proffer treatments that were detrimental to patients. However, the impact of gift-giving has long been of scientific interest (Gouldner, 1960; Levi-Strauss, 1969; Cialdini, 2001) and, unfortunately, such beliefs contradict evidence showing that even the smallest gift (e.g. a can of Coca-Cola) may evoke a desire to repay (Regan, 1971), and that even small, inexpensive gifts and meals can influence doctors’ prescriptions (Katz, Caplan, Merz, 2010; DeJong et al., 2016). Studies have shown that doctors do not perceive themselves as being influenced by gifts they receive (Wazana, 2000; Steinman, Shlipak, McPhee, 2001).
In their discussions of risks, students accepted the idea that companies aim to trigger reciprocation and, although only a few used the correct name for the principle, many described how the reciprocity principle works. This principle can be defined after Robert Cialdini (2001: 20): “we should try to repay, in kind, what another person has provided us”. One student believed that reciprocity is a ‘basic instinct’ and that companies take advantage of people’s natural tendencies to react to the provision of gifts in a predictable way. Bronisław Malinowski ([1922] 1981) observed that the act of exchange as a tool for maintaining positive relations with neighbors has been documented in primitive societies. Additionally, Marcel Mauss ([1950] 2007) suggests that the notion of exchange explains human tendencies to make sacrifices to Gods. It therefore seems reasonable to view reciprocity as being deeply ingrained in human activities. Polish law stipulates that gifts accepted by doctors from medical representatives should not be worth more than 100 PLN, and that they should be related to medical practice (Official Journal of Laws of 2001 No. 126, item 1381). But the theories described above, and the present observations, may lead us to question whether this is the best way to protect doctors from being unduly influenced by the pharmaceutical industry.
The students linked reciprocity not only with gifts and meals, but also with other benefits such as professional growth opportunities and the provision of drug samples, and some students averred the opinion that accepting gifts, even in the form of drug samples, may be ethically dubious. Previous research has shown that Polish doctors and medical students consider contacts with the pharmaceutical industry to be ethically problematic (Stempień, Tkaczyk, 2017). But students’ limited use of the term “conflict of interest” when discussing the pharmaceutical industry (it was only mentioned once) suggests that there might be a gap in their education. Here, although it might be argued that, because the study involved students from different years, some students may have been yet to undergo a medical ethics course, some students had undoubtedly taken such a course. While training in medical ethics is mandatory in the medical curriculum in Poland, there are no standards set for its delivery, and there is no prescription as to whether issues concerning the pharmaceutical industry are covered.
The students noted that gift-giving could lead patients to sustain financial losses. Firstly, because the costs of incentives might be passed on to patients: they might pay more for drugs, and, secondly, because a doctor might prescribe unnecessarily large quantities of drugs or unnecessarily expensive drugs. Such suspicions are at least partially grounded in the scientific literature, which shows that doctors who have close relationships with the pharmaceutical industry are more likely to prescribe more expensive innovative drugs than cheaper generics, even when the advantages of an innovative drug are unproven (Wazana, 2000; Austad, Avorn, Kesselheim, 2011). Also, recent research has shown links between the acceptance of payments and both the prescribing of greater amounts of drugs and an increased likelihood of prescribing more brand-named drugs (Perlis, Perlis, 2016; Modi et al., 2018; Zezza, Bachhuber, 2018; Mitchell et al., 2021).
Loss of patient trust was cited as another risk of doctors cooperating with the pharmaceutical industry. The students thought that when patients saw doctors spending time with PSRs and taking gifts and meals, and when they heard of doctors attending industry-sponsored conferences and courses, this could erode trust in doctors. Such views are likely to be influenced by the Polish media, which often present stories about cooperation in a scandalous vein. The students’ views are also in accordance with David Grande, Judy Shea, and Katrina Armstrong (2012), who stated that relationships between doctors and the pharmaceutical industry frequently lead to lower levels of trust in both doctors and an entire healthcare system. Gift-giving not only undermines trust in doctors, but can also influence patients’ intentions to comply with doctors’ recommendations (Green et al., 2012). Such findings are currently of particular importance given that the Sars-CoV-2 pandemic, which has been ongoing since the end of 2019, has led to a realization that it is vital for public health that people follow the recommendations of experts and believe in the effectiveness of pharmaceutical innovations. Perhaps most importantly in the current context, the lack of trust in both doctors and pharmaceutical industry innovations can lead to an undermining of people’s beliefs in the effectiveness of drugs (e.g. vaccinations) (Razai et al., 2021). Lack of trust might also have other undesirable effects, such as postponing necessary visits to doctors, failure to take medication in accordance with guidelines, and self-medication.
All doctors are obliged to constantly improve their qualifications and gain educational credits (Official Journal of Laws of 2020, item 464). Pharmaceutical companies facilitate the obtaining of credits by sponsoring doctors’ participation in conferences, courses, and training events, and by providing doctors access to scientific literature. The present students mentioned that the pharmaceutical industry is very good at sharing their information, and that their educational materials can be extremely useful. Some students considered all of these aspects of cooperation to be beneficial to patients in that the industry money that goes into educating doctors via these avenues should lead to patients getting better treatment. However, the risk of companies providing biased information was also noted, with some students being convinced that all of the information received from companies needs to be checked. Here, research by Frederick Sierles et al. (2005) showed that most of the medical students they inquired (67.4%) thought that Grand Rounds are often biased to show a sponsoring company’s products in a good light. In the same study, most students (71.3%) agreed with the statement that drug company materials about new drugs are a useful way to learn. The present study depicted the same situation, with students not trusting the pharmaceutical-industry-related information but praising the industry’s educational materials.
In Poland, public and scientific discussion of problems relating to the pharmaceutical industry’s sponsorship of continuing medical education (CME) is a point of interest for sociologists, bioethicists, and journalists, all of whom have identified risks associated with such sponsorship (Makowska, 2010; Polak, 2011; Chańska, 2017a; Jakubiak, 2018; Kaczmarek, 2019; Różyńska et al., 2021). One study has shown that 33% of Polish doctors attend courses, congresses, conferences, or improvement seminars organized by medical facilities or pharmaceutical companies at least once a month (Krzewińska, Olejniczak, Skonieczna, 2015).
Students did not mention anything about the principle of authority (Cialdini, 2001), which is likely to be salient in educational meetings where key opinion leaders give lectures. They did, however, mention the risks of manipulation and loss of patient trust when discussing the industry’s provision of professional growth opportunities to doctors.
Interestingly, students saw doctors’ relationships with PSRs as being beneficial in several respects, with PSRs being said to facilitate doctors’ learning about products, provide agreeable company, and provide opportunities to establish valuable contacts in meetings they invite doctors to. While it would be easy to describe how PSRs are able to utilize all of Robert Cialdini’s principles (Makowska, 2010; Sah, Fugh-Berman, 2013) in manipulating doctors to realize their goals, the liking principle was the most salient in the students’ discussions. Although this principle was not referred to by name, the students described how it functions. The principle states that it is hard for us to say no to people that we like (Cialdini, 2001), and the students mentioned that doctors’ interpersonal contacts with PSRs who have pleasant personalities and who seek to form friendships with them can influence doctors’ prescribing practices.
Another mentioned risk was the loss of time incurred by doctors due to PSRs’ visits (on a aside, it should be noted that Polish law forbids doctors to meet PSRs during working hours (Official Journal of Laws of 2008 No. 210, item 1327), but the present students observed that the law is ineffective and not respected). Loss of time is not an issue uniquely identified by this study, with previous Australian qualitative research by Evan Doran et al. (2006) describing a subset of doctors as avoiding cooperation with the industry for this reason. In the present study, students also linked relationships with PSRs to loss of patient trust and the risk of receiving biased information. This latter suspicion may be well-founded given that a previous study found 11% of statements made by PSRs in short presentations at conferences to be false, but that doctors were unable to recognize this (Ziegler, Lew, Singer, 1995).
The provision of drug samples is a controversial element of pharmaceutical marketing (Institute of Medicine, 2009; Warrier et al., 2010). On the one hand, samples are useful, because, as the students mentioned, they can be distributed to patients in need, doctors can pass them on to the (underfunded) institutions where they work, or they can be used to test whether a drug is suitable for a patient. On the other hand, students believed that providing free drug samples to patients may result in them using the particular drug over the long term. The students also pointed out that doctors may simply retain samples for themselves, in which case the samples become a gift, with all the attendant risks related to gift-giving coming into play. Lisa Chew et al. (2000) have also noted that the availability of drug samples might lead doctors to give them to patients when they would have chosen other drugs if they had not been in possession of the samples. Finally, Richard Adair and Leah Holmgren (2005) showed that resident doctors’ decisions were influenced by them having access to samples, and that they were more likely to prescribe heavily advertised drugs and less likely to use OTC and inexpensive drugs.
Polish discussions about the ethics of pharmaceutical marketing only started at the beginning of the 21st century, and subsequently both journalists and scientists have produced publications and reports dealing with a large range of issues connected with the subject (Lizut, 2006; Polak, 2011; Ozierański, McKee, King, 2012; Chańska, 2017a; Makowska, 2017; Reszka, 2017; Dabiś, 2018; Jakubiak, 2018; Kaczmarek, 2019; Młynarek, 2019) Also, the medical community is becoming open to discussing the topic, with key opinion leaders being involved in these discussions, and with articles appearing in important medical media (Krajewski, 2014; Sławiński, 2014; Sztwiertnia, 2014; Basiewicz, 2015; Radziwiłł, 2015; Chańska, 2016a; 2016b; 2017b; 2017a). Relationships between the pharmaceutical industry and doctors are regulated by the Code of Medical Ethics (Naczelna Izba Lekarska, 2004; Radziwiłł, 2015), and ethics committees have been established in many hospitals, with their number growing dynamically in recent years (Czarkowski, Kaczmarczyk, Szymańska, 2015; Naczelna Izba Lekarska, 2023). Hospitals are also starting to introduce their own internal regulations specifying the appropriate behaviors for doctors to adopt in their relationships with the industry (Szpital Kliniczny im. Wiktora Degi, 2023). Furthermore, the pharmaceutical industry itself has engaged in self-regulation, introducing transparency codes (PZPPF, 2020; INFARMA, 2021) in order to improve ethical standards in the industry’s collaborations with medical staff. Thus, things appear to be moving in a positive direction, but changes are only occurring gradually, often being imposed because of international requirements rather than being the result of grassroots initiatives in Poland.
Students perceived the benefits received from pharmaceutical companies as attractive, and they were seen as important not only for doctors and other medical staff, but also for patients. Students associated at least two risks with each benefit identified in the current study. The research showed that while cooperation with the industry has attractions for students and they provide rationalizations for it (e.g. they expressed the opinion that gift-giving is reasonable due to the poor financial situation of the health service), they also have concerns about cooperation.
The study also revealed that medical students need educating in psychological aspects of cooperation (specifically, ways in which manipulation can occur). While the term ‘reciprocity’ appeared sporadically in discussions, other manipulative techniques were described but not explicitly named. And some principles were not discussed at all. For example, there was no mention of the ‘authority principle’ even though this principle is widely used by the pharmaceutical industry during educational meetings. Also, the term ‘conflict of interest’ appeared only once, and the fact that such a commonly used term was not used more often indicates the need for better tuition in ethical aspects of medical education.
More generally, the present work has shown that it is important that medical students should receive better education in the presently researched aspects of their future careers toward the end of creating a counterbalance between the risks and benefits associated with cooperation between pharmaceutical companies and the medical community.
Cytowanie
Marta Makowska (2023), “It Works by Activating Very Basic Instincts […]”: Medical Students’ Opinions about the Benefits and Risks of the Pharmaceutical Industry’s Marketing Efforts Aimed at Medical Doctors, „Przegląd Socjologii Jakościowej”, t. XIX, nr 3, s. 220–241 (https://doi.org/10.18778/1733-8069.19.3.12).
Adair Richard, Holmgren Leah (2005), Do drug samples influence resident prescribing behavior ? A randomized trial, “The American Journal of Medicine”, vol. 118(8), pp. 881–884, https://doi.org/10.1016/j.amjmed.2005.02.031
Austad Kirsten, Avorn Jerry, Kesselheim Aaron (2011), Medical Students’ Exposure to and Attitudes about the Pharmaceutical Industry: A Systematic Review, “PLOS Medicine”, vol. 8(5), e1001037, https://doi.org/10.1371/journal.pmed.1001037
Basiewicz Andrzej (2015), W sprawie współpracy z przemysłem – czy zawsze zgodnie z prawem?, “Gazeta Lekarska”, vol. 3, p. 50.
Bechoux Lucas, Vleeschouwer Oriane, Vanheuverzwijn Cécile, Verhegghen Florence, Detiffe Alizée, Colle Fabian, Fallon Catherine, Thoreau François (2021), Conflict of interest policies at Belgian medical faculties : Cross - sectional study indicates little oversight, “PLOS ONE”, vol. 16(2), e0245736, https://doi.org/10.1371/journal.pone.0245736
Berger Peter L., Luckmann Thomas (1966), The social construction of reality: A Treatise in the Sociology of Knowledge, London: Penguin Books.
Braun Virginia, Clarke Victoria (2006), Using thematic analysis in psychology, “Qualitative Research in Psychology”, vol. 3(2), pp. 77–101, https://doi.org/10.1191/1478088706qp063oa
Campbell Eric, Weissman Joel, Ehringhaus Susan, Rao Sowmya, Moy Beverly, Feibelmann Sandra, Dorr Goold Susan (2007), Institutional Academic – Industry Relationships, “JAMA”, vol. 298(15), pp. 1779–1786, https://doi.org/10.1001/jama.298.15.1779
Carlat Daniel, Fagrelius Teddy, Ramachandran Reshma, Ross Joseph, Bergh Sallyann (2016), The updated AMSA scorecard of conflict - of - interest policies: A survey of U. S. medical schools, “BMC Medical Education”, vol. 16(1), 202, https://doi.org/10.1186/s12909-016-0725-y
Chańska Weronika (2016a), Konflikt interesów w praktyce lekarskiej – cz. I, http://www.mp.pl/social/article/139701 (accessed: 16.05.2023).
Chańska Weronika (2016b), Konflikt interesów w praktyce lekarskiej – cz. II. Badania naukowe, http://www.mp.pl/social/article/147847 (accessed: 16.05.2023).
Chańska Weronika (2017a), Konflikt interesów w praktyce lekarskiej – cz. III. Konflikty interesów w sferze doskonalenia zawodowego lekarzy, http://www.mp.pl/social/article/155527 (accessed: 16.05.2023).
Chańska Weronika (2017b), Konflikt interesów w praktyce lekarskiej – cz. IV. Tworzenie wytycznych w zakresie praktyki klinicznej oraz wydawanie opinii o finansowaniu świadczeń zdrowotnych z budżetu państwa, http://www.mp.pl/social/article/161967 (accessed: 16.05.2023).
Chew Lisa, O’Young Theresa, Hazlet Thomas, Bradley Katharine, Maynard Charles, Lessler Daniel (2000), Konflikt interesów w praktyce lekarskiej – cz. IV. Tworzenie wytycznych w zakresie praktyki klinicznej oraz wydawanie opinii o finansowaniu świadczeń zdrowotnych z budżetu państwa, “Journal of General Internal Medicine”, vol. 15(7), pp. 478–483, https://doi.org/10.1046/j.1525-1497.2000.08014.x
Cialdini Robert (2001), Influence: Science and Practice, Boston: Allyn and Bacon.
Czarkowski Marek, Kaczmarczyk Katarzyna, Szymańska Beata (2015), Hospital Ethics Committees in Poland, “Science and Engineering Ethics”, vol. 21(6), pp. 1525–1535, https://doi.org/10.1007/s11948-014-9609-x
Dabiś Rozalia (2018), Przedstawiciele medyczni: Wazeliniarstwo jest wręcz obrzydliwe, ale na tym polega ta robota, “Gazeta Wyborcza”, 25th September, https://wyborcza.pl/duzyformat/7,127290,23574300,przedstawiciele-medyczni-wazeliniarstwo-jest-wrecz-obrzydliwe.html (accessed: 16.05.2023).
Dana Jason, Loewenstein George (2003), A Social Science Perspective on Gifts to Physicians From Industry, “JAMA”, vol. 290(2), pp. 252–255, https://doi.org/10.1001/jama.290.2.252
DeJong Colette, Aguilar Thomas, Tseng Chien-Wen, Lin Grace, Boscardin John, Adams Dudley (2016), Pharmaceutical Industry - Sponsored Meals and Physician Prescribing Patterns for Medicare Beneficiaries, “JAMA Internal Medicine”, vol. 176(8), pp. 1114–1122, https://doi.org/10.1001/jamainternmed.2016.2765
Derry Sharon (1999), A Fish Called Peer Learning : Searching for Common Themes, [in:] Angela O’Donnell, Alison King (eds.), Cognitive Perspectives on Peer Learning, New York: Routledge, pp. 197–211.
Doran Evan, Kerridge Ian, McNeill Paul, Henry David (2006), Empirical uncertainty and moral contest: A qualitative analysis of the relationship between medical specialists and the pharmaceutical industry in Australia, “Social Science & Medicine”, vol. 62(6), pp. 1510–1519, https://doi.org/10.1016/j.socscimed.2005.07.037
Giddens Anthony (2006), Sociology, Cambridge: Polity Press.
Gouldner Alvin (1960), The Norm of Reciprocity: A Preliminary Statement, “American Sociological Review”, vol. 25(2), pp. 161–178, https://doi.org/10.2307/2092623
Grabitz Peter, Friedmann Zoe, Gepp Sophie, Hess Leonard Urlich, Specht Lisa, Struck Maja, Tragert Sophie, Walther Tobias, Klemperer David (2020), Quantity and quality of conflict of interest policies at German medical schools: a cross - sectional study and survey, “BMJ Open”, vol. 10(9), e039782, http://doi.org/10.1136/bmjopen-2020-039782
Grande David, Shea Judy, Armstrong Katrina (2012), Pharmaceutical Industry Gifts to Physicians: Patient Beliefs and Trust in Physicians and the Health Care System, “Journal of General Internal Medicine”, vol. 27(3), pp. 274–279, https://doi.org/10.1007/s11606-011-1760-3
Green Michael, Masters Rebeca, James Benjamin, Simmons Bree, Lehman Eric (2012), Do Gifts From the Pharmaceutical Industry Affect Trust in Physicians?, “Family Medicine”, vol. 44(5), pp. 325–231.
INFARMA (2021), Kodeks Dobrych Praktyk INFARMA, Infarma Związek Pracodawców Innowacyjnych Firm Farmaceutycznych, https://www.infarma.pl/etyka/kodeks-dobrych-praktyk/ (accessed: 16.05.2023).
Institute of Medicine (US) Committee on Conflict of Interest in Medical Research, Education, and Practice (2009), Conflict of Interest in Medical Research, Education, and Practice, Washington: National Academies Press.
IQVIA (2023), Rynek farmaceutyczny w 2022 roku, https://www.linkedin.com/posts/iqvia- poland-_rynek- farmaceutyczny-w-2022-roku-podsumowanie-activity-7026538726193258496-sVuI/?utm_source=share&utm_medium=member_desktop (accessed: 16.05.2023).
Jakubiak Luiza (2018), Sponsorowanie edukacji lekarzy przez firmy farmaceutyczne to powielanie biedy polskiej medycyny, https://www.rynekaptek.pl/marketing-i-zarzadzanie/sponsorowanie-edukacji-lekarzy-przez-firmy-farmaceutyczne-to-powielanie-biedy-polskiej-medycyny,29238.html (accessed: 16.05.2023).
Kaczmarek Emilia (2019), Gorzka pigułka. Etyka i biopolityka w branży farmaceutycznej, Warszawa: Wydawnictwo Naukowe Scholar.
Katz Dana, Caplan Arthur, Merz Jon (2010), All Gifts Large and Small: Toward an Understanding of the Ethics of Pharmaceutical Industry Gift - Giving, “The American Journal of Bioethics”, vol. 10(10), pp. 11–17, https://doi.org/10.1080/15265161.2010.519226
King Marissa, Essick Connor, Bearman Peter, Cole Jonathan, Ross Joseph (2013), Medical school gift restriction policies and physician prescribing of newly marketed psychotropic medications: Difference - in - differences analysis, “BMJ”, no. 346, f264, https://doi.org/10.1136/bmj.f264
Krajewski Romuald (2014), Kodeks Przejrzystości EFPIA / INFARMA. Komentarz, “Gazeta Lekarska”, vol. 8/9, p. 33.
Krzewińska Dagmara, Olejniczak Dominik, Skonieczna Joanna (2015), Problemy i bariery w procesie kształcenia zawodowego lekarzy – badanie własne, „Medycyna Ogólna i Nauki o Zdrowiu”, vol. 21(3), pp. 332–337, https://doi.org/10.5604/20834543.1165363
Leonardo Alves Teresa, Lexchin Joel, Mintzes Barbara (2019), Medicines Information and the Regulation of the Promotion of Pharmaceuticals, “Science and Engineering Ethics”, vol. 25(4), pp. 1167–1192, https://doi.org/10.1007/s11948-018-0041-5
Levi-Strauss Claude (1969), The Elementary Structures of Kinship, Boston: Beacon Press.
Lieb Klaus, Brandtönies Simone (2010), A Survey of German Physicians in Private Practice About Contacts With Pharmaceutical Sales Representatives, “Deutsches Ärzteblatt International”, vol. 107(22), pp. 392–398, https://doi.org/10.3238/arztebl.2010.0392
Lieb Klaus, Koch Cora (2013), Medical Students’ Attitudes to and Contact With the Pharmaceutical Industry, “Deutsches Ärzteblatt International”, vol. 110(35–36), pp. 584–590, https://doi.org/10.3238/arztebl.2013.0584
Lizut Mikołaj (2006), Jak ustawić lekarza, “Gazeta Wyborcza”, vol. 120, p. 4.
Makowska Marta (2010), Etyczne standardy marketingu farmaceutycznego, Warszawa: Wydawnictwo CeDeWu.
Makowska Marta (2017), Polish physicians’ cooperation with the pharmaceutical industry and its potential impact on public health, “PLOS ONE”, vol. 12(9), e0184862, https://doi.org/10.1371/journal.pone.0184862
Makowska Marta (2022), How polish medical students are socialised to cooperate with the pharmaceutical industry: a focus group study of the importance of informal, hidden and null curricula, “Health Sociology Review”, vol. 31(1), pp. 81–95, https://doi.org/10.1080/14461242.2021.1899842
Makowska Marta, Nowakowski Michał (2020), Leki jako specyficzne dobro konsumpcyjne. Czynniki wpływające na wzrost konsumpcji farmaceutyków – perspektywa socjologiczna, “Konteksty Społeczne”, vol. 8(2), pp. 206–233.
Makowska Marta, Kaczmarek Emilia, Rodzinka Marcin (2022), Transparency or restricting gifts ? Polish medical students’ opinions about regulating relationships with pharmaceutical sales representatives, “Monash Bioethics Review”, vol. 40, pp. 49–70, https://doi.org/10.1007/s40592-021-00128-2
Malinowski Bronisław ([1922] 1981), Dzieła: Argonauci Zachodniego Pacyfiku, Warszawa: Państwowe Wydawnictwo Naukowe.
Mason Paul, Tattersall Martin (2011), Conflicts of interest: A review of institutional policy in Australian medical schools, “The Medical Journal of Australia”, vol. 194(3), pp. 121–125, https://doi.org/10.5694/j.1326-5377.2011.tb03796.x
Mauss Marcel ([1950] 2007), Szkic o darze. Forma i podstawa wymiany w społeczeństwach archaicznych, [in:] Ewa Nowicka, Małgorzata Głowacka-Grajper (eds.), Świat człowieka, świat kultury, Warszawa: Wydawnictwo Naukowe PWN, pp. 107–168.
McCormick Brendan, Tomlinson George, Brill-Edwards Patric, Detsky Allan (2001), Effect of Restricting Contact Between Pharmaceutical Company Representatives and Internal Medicine Residents on Posttraining Attitudes and Behavior, “JAMA”, vol. 286(16), pp. 1994–1999, https://doi.org/10.1001/jama.286.16.1994
Merton Robert, Kitt Alice (1950), Contributions to the Theory of Reference Group Behavior, [in:] Robert Merton, Paul Lazarsfeld (eds.), Continuities in Social Research, Glencoe: Free Press, pp. 40–105.
Mitchell Aaron, Trivedi Niti, Gennarelli Renee, Chimonas Susan, Tabatabai Sara, Goldberg Johanna, Diaz Luis, Korenstein Deborath (2021), Are Financial Payments From the Pharmaceutical Industry Associated With Physician Prescribing? A Systematic Review, “Annals of Internal Medicine”, vol. 174(3), pp. 353–361, https://doi.org/10.7326/M20-5665
Młynarek Magdalena (2019), Zjawiska patologiczne na rynku farmaceutycznym, Warszawa: Instytut Wymiaru Sprawiedliwości, https://iws.gov.pl/wp-content/uploads/2020/03/IWS_M%C5%82ynarek-M._Zjawiska-patologiczne-na-rynku-farmaceutycznym.pdf (accessed: 16.05.2023).
Modi Parth, Wang Ye, Kirk Peter, Dupree James, Singer Eric, Chang Steven (2018), The Receipt of Industry Payments is Associated With Prescribing Promoted Alpha - blockers and Overactive Bladder Medications, “Urology”, vol. 117, pp. 50–56, https://doi.org/10.1016/j.urology.2018.04.008
Naczelna Izba Lekarska (2004), Kodeks Etyki Lekarskiej, https://nil.org.pl/dokumenty/kodeks-etyki-lekarskiej (accessed: 16.05.2023).
Naczelna Izba Lekarska (2023), Szpitalne komisje etyczne, https://nil.org.pl/dzialalnosc/osrodki/osrodek-bioetyki/szpitalne-komisje-etyczne (accessed: 16.05.2023).
Ozierański Piotr, McKee Martin, King Lawrence (2012), Pharmaceutical lobbying under postcommunism: Universal or country - specific methods of securing state drug reimbursement in Poland?, „Health Economics, Policy and Law”, vol. 7(2), pp. 175–195, https://doi.org/10.1017/S1744133111000168
Paice Elisabeth, Heard Shelley, Moss Fiona (2002), How important are role models in making good doctors?, “BMJ”, vol. 325(7366), pp. 707–710, https://doi.org/10.1136/bmj.325.7366.707
Perlis Roy, Perlis Clifford (2016), Physician Payments from Industry Are Associated with Greater Medicare Part D Prescribing Costs, “PLOS ONE”, vol. 11(5), e0155474, https://doi.org/10.1371/journal.pone.0155474
Polak Paulina (2011), Nowe formy korupcji. Analiza socjologiczna sektora farmaceutycznego w Polsce, Kraków: Zakład Wydawniczy “Nomos”.
PZPPF (2020), Kodeks Etyczny 2020 Medicines for Europe, https://drive.google.com/file/d/1irwojHac70B8uMATPimiBp65orDjuNwg/view?usp=share_link&usp=embed_facebook (accessed: 16.05.2023).
Radziwiłł Konstanty (2015), Rozdział IIa Kodeksu etyki lekarskiej – związki lekarzy z przemysłem, “Medyczna Wokanda”, vol. 7(7), pp. 31–36.
Razai Mohammad, Chaudhry Umar, Doerholt Katja, Bauld Linda, Majeed Azeem (2021), Covid - 19 vaccination hesitancy, “BMJ”, vol. 373, n1138, https://doi.org/10.1136/bmj.n1138
Regan Dennis (1971), Effects of a favor and liking on compliance, “Journal of Experimental Social Psychology”, vol. 7(6), pp. 627–639, https://doi.org/10.1016/0022-1031(71)90025-4
Reszka Paweł (2017), Mali bogowie, Warszawa: Wydawnictwo Czerwone i Czarne.
Różyńska Joanna, Chańska Weronika, Łuków Paweł, Zielińska Eleonora, Chyrowicz Barbara, Karkowska Dorota, Hartman Jan (2021), Konflikt interesów w praktyce zawodów medycznych. Rekomendacje Komisji do spraw etyki w ochronie zdrowia ( 2013–2016 ), “Przegląd Prawa Medycznego”, vol. 3(1–2), pp. 5–32.
Sah Sunita, Fugh-Berman Adriane (2013), Physicians under the Influence: Social Psychology and Industry Marketing Strategies, “The Journal of Law, Medicine & Ethics”, vol. 41(3), pp. 665–672, https://doi.org/10.1111/jlme.12076
Scheffer Paul, Guy-Coichard Christian, Outh-Gauer David, Calet-Froissart Zoéline, Boursier Mathilde, Mintzes Barbara, Borde Jean-Sébastien (2017), Conflict of Interest Policies at French Medical Schools: Starting from the Bottom, “PLOS ONE”, vol. 12(1), e0168258, https://doi.org/10.1371/journal.pone.0168258
Shnier Adrienne, Lexchin Joel, Mintzes Barbara, Jutel Anne Marie, Holloway Kelly (2013), Too Few, Too Weak: Conflict of Interest Policies at Canadian Medical Schools, “PLOS ONE”, vol. 8(7), e68633, https://doi.org/10.1371/journal.pone.0068633
Sierles Frederick, Brodkey Amy, Cleary Lynn, McCurdy Frederick, Mintz Matthew, Frank Julia, Joanne Lynn, Chao Jason, Morgenstern Bruce, Shore William, Woodard John (2005), Medical Students’ Exposure to and Attitudes About Drug Company Interactions: A National Survey, “Journal of the American Medical Association”, vol. 294(9), pp. 1034–1042, https://doi.org/10.1001/jama.294.9.1034
Sławiński Piotr (2014), W sprawie współpracy z przemysłem, “Gazeta Lekarska”, vol. 11, p. 52.
Steinman Michael, Shlipak Michael, McPhee Stephen (2001), Of principles and pens: Attitudes and practices of medicine housestaff toward pharmaceutical industry promotions, “The American Journal of Medicine”, vol. 110(7), pp. 551–557, https://doi.org/10.1016/s0002-9343(01)00660-x
Stempień Jakub, Tkaczyk Marcin (2017), Lekarze i studenci medycyny wobec sytuacji etycznie trudnych. Komunikat z badań, “Władza Sądzenia”, vol. 12, pp. 90–99.
Stewart David, Shamdasani Prem, Rook Dennis (2007), Focus groups: Theory and practice, Thousand Oaks: Sage Publications.
Szpital Kliniczny im. Wiktora Degi (2023), Procedura postępowania antykorupcyjnego w kontaktach pracowników z przedstawicielami: firm farmaceutycznych, producentów i dostawców wyrobów medycznych oraz innych dostawców towarów i usług w ortopedyczno - rehabilitacyjnym Szpitalu Klinicznym im. Wiktora Degi Uniwersytetu Medycznego im. Karola Marcinkowskiego w Poznaniu, https://orsk.pl/wp-content/uploads/2020/08/20200629_antykorupcja_procedura.pdf (accessed: 16.05.2023).
Sztwiertnia Paweł (2014), Przejrzystość nowym standardem, “Gazeta Lekarska”, vol. 8/9, p. 32.
Warrier Rugmini, Monaghan Michael, Maio Anna, Huggett Kathryn, Rich Eugene (2010), Effect of drug sample availability on physician prescribing behavior: A systematic review, “Clinical Reviews and Opinions”, vol. 2(4), pp. 41–48.
Wazana Ashley (2000), Physicians and the Pharmaceutical Industry. Is a Gift Ever Just a Gift?, “JAMA”, vol. 283(3), pp. 373–380, https://doi.org/10.1001/jama.283.3.373
Zboroń Halina (2009), Konstruktywizm społeczny – nowe teoretyczne podejście w badaniach nad gospodarką, “Prakseologia”, vol. 149, pp. 63–87.
Zezza Mark, Bachhuber Marcus (2018), Payments from drug companies to physicians are associated with higher volume and more expensive opioid analgesic prescribing, “PLOS ONE”, vol. 13(12), e0209383, https://doi.org/10.1371/journal.pone.0209383
Ziegler Michael, Lew Pauline, Singer Brian (1995), The Accuracy of Drug Information From Pharmaceutical Sales Representatives, “JAMA”, vol. 273(16), pp. 1296–1298, https://doi.org/10.1001/jama.1995.03520400066047

