Chemical Characterization of Archaeological Pulp Stones from 18th–19th Century Radom, Poland: A Pilot Study
Jacek Tomczyk
 https://orcid.org/0000-0002-0605-665X
https://orcid.org/0000-0002-0605-665X
Institute of Biological Sciences, Cardinal Stefan Wyszyński University in Warsaw, Poland
Piotr Regulski
 https://orcid.org/0000-0002-3692-3582
https://orcid.org/0000-0002-3692-3582
Department of Dental and Maxillofacial Radiology, Medical University of Warsaw, Poland
Katarzyna Góralczyk
 https://orcid.org/0000-0002-9839-2449
https://orcid.org/0000-0002-9839-2449
Institute of Biological Sciences, Cardinal Stefan Wyszyński University in Warsaw, Poland
Grzegorz Tylko
 https://orcid.org/0000-0002-6669-0142
https://orcid.org/0000-0002-6669-0142
Department of Cell Biology and Imaging, Institute of Zoology and Biomedical Research, Faculty of Biology, Jagiellonian University, Poland
Abstract. The purpose of this study was to evaluate the calcium (Ca) and phosphorus (P) contents of pulp stones in a historical population (18th–19th centuries) from Radom, Poland. Ten molars from adults from the Radom cemetery (18th–19th centuries) were used in the study. The crowns of the teeth were mechanically opened, and visible pulp stones were examined with a scanning electron microscope equipped with a Si(Li) energy dispersive detector (energy dispersive X-ray spectrometry detector, which uses a lithium (Li)-doped silicon (Si) single-crystal semiconductor as a detector element). Enamel presented higher values of Ca/P ratio compared with the dentine and/or pulp stones. Moreover, in one case, the external layer of the pulp stone had significantly lower Ca and P contents compared with the internal layer (Ca: 13.97±0.31 vs. 34.2±0.37 [wt%]; P: 6.13±0.21 vs. 16.5±0.34 [wt%]). Evaluation of the Ca and P composition of the analyzed enamel and dentine in the Radom samples from the 18th–19th centuries produced findings that are consistent with studies conducted on teeth from contemporary populations. In the case of one individual, two chemically distinct layers were diagnosed in the pulp stone. It is difficult to interpret this finding based on a single case, but it may be due to the fact that the outer layer is characterized by higher organic structure.
Keywords: pulp stones, calcium, phosphorus, mineralization
Pulp stones are calcified structures that occur in the coronal and radicular pulp, where they may be free, attached, or embedded in the dentine. Free pulp stones are present within the pulp tissue proper and are the most commonly seen type on radiographs (Goga et al. 2008; Al-Ghurabi and Najm 2012). Pulp stones can be observed in deciduous and permanent dentition. Moreover, pulp stones can occur in both erupted and unerupted teeth, as supported by previous studies (Şener et al. 2009; Çolak et al. 2012; Kaabi et al. 2024).
The exact cause and mechanism of pulp calcification are still largely unknown and have been the subject of a great debate (Mitręga and Dreher 1968; Barnaś et al. 1972; Goga et al. 2008; Marshall et al. 2023). Among the main etiological factors mentioned in the literature are: the age of the individual, trauma, periodontal disease, dental caries, abrasion, and genetic predisposition (Ozkalayci et al. 2011; Bahetwar et al. 2012; Çolak et al. 2012). Diseases of the metabolic and/or cardiovascular systems may also co-occur with pulp stones (Huang and Chen 2016; Nicklisch et al. 2021). In our previous study (Tomczyk et al. 2017), we showed that pulp calcification is not connected with the age of the individual; rather, it is caused primarily by pulp irritation. This irritation may be the result of either mechanical factors (dental wear) or disease processes (dental caries and periodontal diseases). The type of diet is also discussed regarding the formation of pulp stones (Raj et al. 2012; Tomczyk et al. 2014). Nicklisch et al. (2021) showed that individuals with higher δ15N isotope values in their bone collagen appear to be more frequently affected by pulp stones. This finding implies that diets containing higher amounts of animal protein may influence the formation and development of pulp stones.
In dental examinations, the most common pulp stones are diagnosed using intraoral radiography. However, this applies only to structures that exceed 200 μm in diameter. Smaller pulp stones are diagnosed using cone-beam computed tomography (Gulsahi et al. 2009; Sisman et al. 2012). In selected cases, the diagnosis of pulp stones can be based on histological or micro-CT analysis. However, the examination procedure in both methods is time-consuming and costly (Goga et al. 2008; Marshall et al. 2023). The evaluation of pulp stones increasingly uses advanced technology, such as inductively coupled plasma atomic emission spectroscopy (ICP-AES), which makes it possible to analyze their structure and chemical properties. This method is based on the excitation of atoms and ions with plasma. The result is the emission of electromagnetic radiation at wavelengths specific to the element (Milcent et al. 2019). The results from this evaluation have confirmed earlier reports that the density and chemical composition of pulp stones is very similar to dentin (Mitręga and Dreher 1968; Barnaś et al. 1972), mainly regarding the contents of the two main elements, calcium (Ca) and phosphorus (P) (Le May and Kaqueler 1993).
The presence of pulp stones as well as their structure and composition are a topic of clinical research (Turkal et al. 2013; Gaddalay et al. 2015; Palatyńska-Ulatowska et al. 2021; Mirah et al. 2023). This is because knowledge of pulp stones is important in endodontic treatment. Unfortunately, only a few studies have addressed pulp stones in historical populations. The authors of these papers have typically focused on the occurrence of pulp stones and their location (Elvery et al. 1998; Tomczyk et al. 2014; Tomczyk et al. 2017; Nicklisch et al. 2021). There are several reasons for the limited number of studies on pulp stones in historical populations. First, it is impossible to subject all specimens to radiography; usually, only random samples of specimens are examined. Second, highly specialized evaluations are invasive in the sense that they require mechanical opening of the tooth chamber, which leads to permanent damage to the tooth. The historical value of archaeological materials prevents conducting such analyses. Therefore, only certain materials from archaeological sites can be subjected to such examination (Nicklisch et al. 2021). Such an opportunity to study pulp stones has arisen for several specimens from a historical cemetery from Radom (Poland).
The present study evaluated the Ca and P contents in the pulp stones of individuals from a historical population from Radom (18th–19th centuries). This study aimed to answer the following research questions: (i) Do the Ca and P contents vary among the studied dental structures? (ii) Do the pulp stones exhibit differences in Ca and P content between their outer and inner layers?
Materials and methods
Dental material of adult individuals from the historical population of Radom (Poland), which came from the cemetery used in the years 1791–1811, was selected for the study. The dental material used for the research is stored at the Institute of Biological Sciences, Cardinal Stefan Wyszynski University in Warsaw. Material was stored in a dry place, stored from possible contamination that could affect the results of the study. The material used in the present study had been analyzed previously in terms of the frequency and location of pulp stones (Tomczyk et al. 2017). The presence of pulp stones was determined with a portable X-ray machine (EZX-60, Edlen Imaging, USA). Due to the invasive (destructive) nature of the study, 10 molars were selected from the described material for further analysis.
First, crowns of the teeth were opened with a dental drill. Next, the edges of the crowns were filed down so that there were leading-edge pulp stones. The transversely cut teeth with the pulp chamber filled with pulp stones were polished gently with sandpaper and rinsed in an ultrasonic bath in cold deionized water. After air drying, each tooth specimen was mounted in a microscopic holder with carbon conductive paint (EM-Tec C33, Rave Scientific, USA) and stored under vacuum overnight. The exposed surfaces of enamel, dentine, and pulp stones were positioned parallel to the holder surface. After coating with a thin layer of carbon (JEC-530, JEOL, Tokyo, Japan), the samples were observed in scanning electron microscope (SEM) (JSM-5410, JEOL) equipped with an Si(Li) energy-dispersive detector (EDS; Noran Instruments Inc., the Netherlands). To determine qualitatively the elemental composition of all dental structures, X-ray microanalysis was performed with the following conditions: an accelerating voltage of 15 keV and an absorption current of 100 pA as measured for the aluminum stub, and a 25° take-off angle for the EDS detector, which was positioned 30 mm from the specimen to achieve a 0.033 sr solid angle. Each analysis was performed in the raster mode at 750× magnification to obtain the total X-ray intensities emitted from the enamel, dentine, and pulp stones not exceeding 30% of the EDS detector deadtime. The same analytical conditions were used for quantitative estimation of the Ca and P contents. For this endeavor, the apatite standard was measured (SPI Supplies, West Chester, PA, USA).
Three measurements were taken at random locations (the test locations were estimated visually) on each tested dental structure (enamel and dentin). In addition, three random measurements (averaged before the statistical analysis) were taken from the pulp stones from the central part (internal layer) and the periphery (external layer). All spectra were processed to obtain the peak-to-background (P/B) Ca and P values (Roomans 1988). Next, the P/B values derived from the apatite standard were compared with the P/B values for unknown enamel, dentine, and pulp stones and the Ca and P contents [wt%] were calculated. Differences in the Ca and P contents as well as the Ca/P ratio were estimated using the non-parametric Kruskal–Wallis test (Statistica, TIBCO Software Inc., Palo Alto, CA, USA). The Shapiro–Wilk test was used to test for normality. A p-value <0.05 was considered to indicate a statistically significant difference.
Results
X-ray microanalysis of three parts of teeth – enamel, dentine, and pulp stones – revealed no significant differences in the Ca and P contents (Table 1).
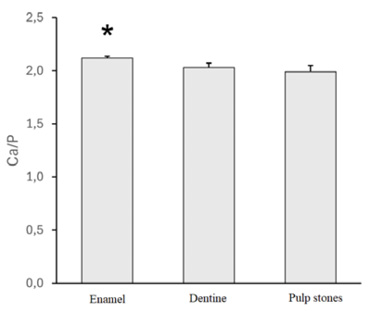
The enamel part was the most mineralized, with a notably higher continuum radiation (background) intensity compared with the dentine and pulp stones (Fig. 1). This effect is due to the strong contribution of elements with a higher atomic number, that is, Ca (Z=20), in the generation of bremsstrahlung rather than elements of organic matter, that is, H (Z=1), C (Z=6), N (Z=7), or O (Z=8) (Goldstein et al., 2018). Indeed, enamel presented a significantly higher Ca/P ratio compared with the dentine or pulp stones (p=0.048) (Fig. 2).
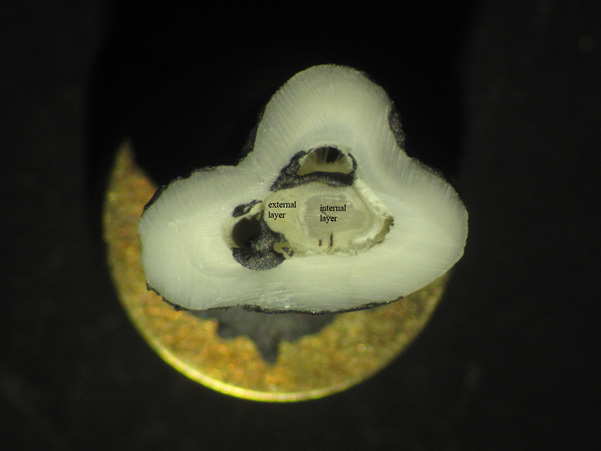
Although the Kruskal–Wallis test revealed no statistically significant differences in calcium (Ca) and phosphorus (P) contents individually among enamel, dentine, and pulp stones (Table 1), the Ca/P ratio differed significantly. This apparent discrepancy can be explained by the cumulative effect of even minor, statistically insignificant variations in Ca and P contents, which collectively affect their proportional relationship, resulting in a significant difference in the Ca/P ratio. In one case, the pulp stone showed two layers that had a distinct elemental composition. The external layer had markedly lower Ca and P contents compared with the internal layer (Ca: 13.97±0.31 vs. 34.2±0.37 [wt%]; P: 6.13±0.21 vs. 16.5±0.34 [wt%]) (Fig. 3). The other pulp stones did not show such variation.
| no data | Amount [%wt] | no data | ||
|---|---|---|---|---|
| Elements | Enamel | Dentine | Pulp stones | p-value* |
| P | 16.41±0.78 | 16.55±0.64 | 16.71±0.73 | 0.0796 |
| Ca | 35.43±1.72 | 33.51±0.84 | 33.36±1.20 | 0.0685 |
*Kruskal–Wallis statistical analysis
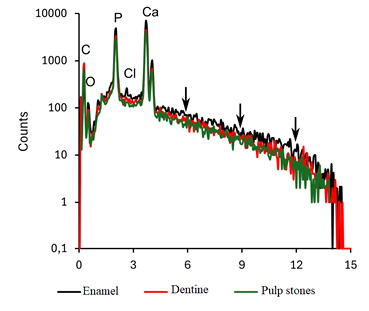
Discussion
Ca and P are the main elements that make up the structure of teeth, so these elements have been widely evaluated (Arnold and Gaengler 2007; He et al. 2011; Liu et al. 2013; Palatyńska-Ulatowska et al. 2021; Sarna-Boś et al. 2022); however, the authors of these studies focused on contemporary populations. Murray (1936) reported a Ca/P ratio variation for human teeth that varied from 1.92 (dentin) to 2.15 (enamel). Hillson (1996) found that the biogenic Ca/P ratio varied from 1.91 to 2.17 for enamel and from 2.1 to 2.2 for dentin. Different Ca/P ratios indicate the different nature of enamel and dentin (Arnold and Gaengler 2007). This ratio decreases in the case of demineralization of enamel due to dental caries (Robinson et al. 1995; Sakoolnamarka et al. 2005). The Ca and P contents of the analyzed teeth from the 18th–19th centuries in Radom are consistent with studies conducted on teeth from modern populations (Arnold and Gaengler 2007; He et al. 2011; Sarna-Boś et al. 2022). Moreover, the Ca/P ratios calculated for the enamel and dentin are within the range of variation of this indicator for contemporary populations (Hillson 1996; Sarna-Boś et al. 2022). The content of elements in teeth can fluctuate due to, among other things, a diverse diet, which is the source of these elements. This means that in different historical periods, when there were different dietary trends, the elemental content of teeth can vary (e.g. Nedoklan et al. 2021). The elemental composition, including Ca and P, is also influenced by the environmental conditions in which the skeletal materials were deposited. For example, alkaline soil better protects bones and teeth compared with acidic soil, which easily destroys hydroxyapatite (Stipisic et al. 2014). The lack of differences in the abovementioned studies may be due to a similar diet for the current population as well as the population in the 18th–19th centuries. This may be confirmed by other anthropological studies (Pach et al. 2023; Perkowski et al. 2024).
The Ca/P ratio did not differ significantly between the dentin and pulp stones. However, there was a significant difference between the enamel and the dentin and pulp stones (Fig. 2). These results indicate that pulp stones have chemical properties that are similar to dentin, a finding that is consistent with the results of other studies conducted on teeth extracted from modern patients (Milcent et al. 2019). Consistently, Le May and Kaqueler (1993) pointed out that pulp stones chemically resemble dentin, with average Ca and P contents of 32% and 15%, respectively. Milicent et al. (2019) found differences between pulp stones and dentin. They noted that pulp stones are characterized by the presence of water and more organic material. These features observed on the teeth from contemporary patients were also captured in the historical population.
Of note, one pulp stone showed two distinctly different areas (the external and internal layers) based on the Ca and P contents. Pulp stones of the other individuals did not show chemical differentiation of their layers. Milcent et al. (2019) made similar observations with teeth that were extracted from patients. According to this study the inner surface of pulp stones shows increased levels of oxygen, calcium, and phosphorus compared to the outer surface which might be attributed to higher organic structure. It is well known that pulp stones show very high heterogeneity in their structure and composition, so it is difficult to make far-reaching interpretations on the basis of a single case (Berès et al. 2016).
Limitations
This study has its limitations. The main limitation of most historical population studies is their small size. As mentioned, the examinations performed are destructive in nature, as they require mechanical opening of the tooth chamber. Such a procedure cannot be standardly performed on historical materials.
Contribution to the field
There is still controversy over both the causes and mechanisms of the formation of pulp stones. This issue is important for several reasons, including for endodontic treatment. While research on the teeth of modern patients is common, examination of odontological material from histological specimens is extremely rare. The results from the present study confirm the observation that pulp stones are chemically similar to dentin. This means that dentin irritation is an important cause of pulp stone formation. In addition, the formation of pulp stones occurs via a certain process that involves the mineralization and calcification of these structures. This can be useful in the treatment process when assessing the degree of development of pulp stones in the tooth.
Conclusion
Studies of pulp stones from historical populations are not often carried out due to the uniqueness and specificity of the dental material. In the present study, we analyzed the chemical composition of pulp stones; it corresponded to the chemical composition of dentin. Enamel showed the most mineralized structure, as the intensity of continuous (background) radiation was significantly higher compared with the dentin and pulp stones. In one pulp stone, we noted two chemically distinct areas (the internal and external layers). Further analysis of larger samples is needed to assess whether such layering is a common feature in historical pulp stones.
References
Al-Ghurabi ZH, Najm AA. 2012. Prevalence of pulp stone (Orthopantomographic-based). JBCD 24:80–4.
Arnold WH, Gaengler P. 2007. Quantitative analysis of the calcium and phosphorus content of developing and permanent human teeth. Ann Anat 189:183–90. https://doi.org/10.1016/j.aanat.2006.09.008
Bahetwar SK, Pandey RK, Singh RK, Bahetwar TS, Wahid A. 2012. A biochemical and histopathological evaluation of generalized pulp calcification in young permanent teeth. Indian. J Dent Res 23:123. https://doi.org/10.4103/0970-9290.99062
Barnaś E, Chlebowska M, Hoehne T. 1972. Dentinomas. Czas Stomatol 25(12):1205–12.
Berès F, Isaac J, Mouton L, Rouzière S, Berdal A, Simon S, Dessombz A. 2016. Comparative physicochemical analysis of pulp stone and dentin. J Endodont 42(3):432–38. https://doi.org/10.1016/j.joen.2015.11.007
Çolak H, Çelebi AA, Hamidi MM, Bayraktar Y, Çolak T, Uzgur R. 2012. Assessment of the prevalence of pulp stones in a samples of Turkish central Anatolian population. Sci World J. https://doi.org/10.1100/2012/804278
Elvery MW, Savage NW, Wood WB. 1998. Radiographic study of the Broadbeach aboriginal dentition. Am J Phys Anthropol 107:211–19. https://doi.org/10.1002/(SICI)1096-8644(199810)107:2<211::AID-AJPA7>3.0.CO;2-X
Gaddalay S, Pathan M, Kale A, Ahhirao Y. 2015. Prevalence of pulp stones in urban and rural population of Latur Maharashtra and the challenges encountered: an endodontic perspectives. J Dent Med Sci 9:5–8. https://doi.org/10.9790/0853-14930508
Goga R, Chandler NP, Oginni AO. 2008. Pulp stones: a review. Int Endodont J 41:457–468. https://doi.org/10.1111/j.1365-2591.2008.01374.x.
Goldstein JI, Newbury DE, Joy DC, Lyman CE, Echlin P, Lifshin E, Sawyer E, Michael JR. 2018. Scanning Electron Microscopy and X-ray Microanalysis. New York: Plenum Press.
Gulsahi A, Cebeci AI, Özden S. 2009. A radiographic assessment of the prevalence of pulp stones in a group of Turkish dental patients. Int Endodont J 42:735–39. https://doi.org/10.1111/j.1365-2591.2009.01580.x
He B, Huang S, Zhang C, Jing J, Hao Y, Xiao L, Zhou X. 2011. Mineral densities and elemental content in different layers of healthy human enamel with varying teeth age. Arch Oral Biol 56:997–1004. https://doi.org/10.1016/j.archoralbio.2011.02.015
Hillson S. 1996. Dental anthropology. Cambridge: Cambridge University Press.
Huang LG, Chen G. 2016. A histological and radiographic study of pulpal calcification in periodontally involved teeth in a Taiwanese population. J Dent Sci 11(4):405–10. https://doi.org/10.1016/j.jds.2016.05.001
Kaabi HH, Riyahi AM, Bakrman AK, Almutaw YA, Alrumayyan SF, Al-Maflehi NS. 2024. Pulp stones in unerupted teeth: a retrospective analysis using cone-beam computed tomography. BMC Oral Health 24:714. https://doi.org/10.1186/s12903-024-04503-3
Le May O, Kaqueler C. 1993. Electron probe micro-analysis of human dental pulp stones. J Scan Micros 7:267–72.
Liu H-Y, Chao J-H, Chuang C-Y, Chiu H-L, Yang C-W, Sun Y-C. 2013. Study of P, Ca, Sr, Ba and Pb levels in enamel and dentine of human third molars for environmental and archaeological research. Adv Anthropol 3(2):71–7. https://doi.org/10.4236/aa.2013.32010
Marshall G, Verdelis K, Peters OA, 2023. Morphology of pulpal mineralizations: A scoping review. J Dent. https://doi.org/10.1016/j.jdent.2023.104745
Milcent CPF, Da Silva TG, Baika LM, Grassi MT, Carneiro E, Franco A, De Lima AAS. 2019. Morphologic, structural, and chemical properties of pulp stones in extracted human teeth. J Endodont 45(12):1504–12. https://doi.org/10.1016/j.joen.2019.09.009
Mirah MA, Bafail A, Shaheen S, Baik A, Zaid BA, Alharbi A, Alahmadi O. 2023. Assessment of pulp stones among western Saudi populations: A Cross-Sectional study. Cureus 15(9): e46056. https://doi.org/10.7759/cureus.46056
Mitręga J, Dreher W. 1968. Calcifying changes in the pulp. Czas Stomatolo 21(11):1325–31.
Murray MM. 1936. The Chemical composition of teeth. IV. The calcium, magnesium and phosphorus contents of the teeth of different animals. A brief consideration of the mechanism of calcification. London: University of London.
Nedoklan S, Knezovic Z, Knezovic N, Sutlovic D. 2021. Nutritional and mineral content in human teeth through the centuries. Arch Oral Biol 124:105075. https://doi.org/10.1016/j.archoralbio.2021.105075
Nicklisch N, Schierz O, Enzmann F, Knipper C, Held P, Vach W, Dresely V, Meller H, Friederich S, Alt KW. 2021. Dental pulp calcifications in prehistoric and historical skeletal remains. Ann Anat 235. https://doi.org/10.1016/j.aanat.2021.151675
Ozkalayci N, Zengin AZ, Turk SE, Sumer AP, Bulucu B, Kirtiloglu T. 2011. Multiple pulp stones: a case report. Eur J Dent 5:210–14.
Pach J, Regulski P, Tomczyk J, Reymond J, Osipowicz K, Strużycka I. 2023. Prevalence of taurodontism in contemporary and historical populations from Radom: a biometric analysis of radiological data. J Clin Med. https://doi.org/10.3390/jcm12185988
Palatyńska-Ulatowska A, Fernandes MC, Pietrzycka K, Koprowicz A, Klimek L, Souza RA, Pradebon M, de Figueiredo JAP. 2021. The pulp stones: morphological analysis in scanning electron microscopy and spectroscopic chemical quantification. Medicina (B Aires) 58(1). https://doi.org/10.3390/medicina58010005
Perkowski K, Marczyńska-Stolarek M, Regulski P, Tomczyk J. 2024. Characteristics of dental malocclusion in a 18th/19th century population from Radom (Poland). Int J Paleopathol 47:21–6. https://doi.org/10.1016/j.ijpp.2024.09.001
Raj AC, Jayaprasad A, Manasa A, Darshan DD, Seema M, Ambili A. 2012. Correlation of pulp stone prevalence with dietary habits – A pilot study. Health Sci 1(3):JS003C.
Robinson C, Kirkham J, Brookes SJ, Shore R. 1995. Chemistry of mature enamel. Chapter 8. In: C Robinson, J Kirkham, editors. Dental Enamel – Formation to Destruction. Boca Raton CRC Press. 167–88.
Roomans GM. 1988. Quantitative X-ray microanalysis of biological specimens. J Electron Micr Tech 9:19–43. https://doi.org/10.1002/jemt.1060090104
Sakoolnamarka R, Burrow MF, Swain M, Tyas MJ. 2005. Microhardness and Ca:P ratio of carious and CarisolvTM treated caries-affected dentine using an ultra-micro-indentation system and energy dispersive analysis of x-rays – A pilot study. Aust Dent J 50(4):246–50. https://doi.org/10.1111/j.1834-7819.2005.tb00368.x
Sarna-Boś K, Boguta P, Skic K, Wiącek D, Maksymiuk P, Sobieszczański J, Chałas R. 2022. Physicochemical properties and surface characteristics of ground human teeth. Molecules 27:5852. https://doi.org/10.3390/molecules27185852
Şener S, Cobankara FK, Akgunlu F. 2009. Calcifications of the pulp chamber: prevalence and implicated factors. Clin Oral Invest 13:209–15. https://doi.org/10.1007/s00784-008-0212-x
Sisman Y, Aktan AM, Tarim-Ertas E, Çiftçi ME, Şekerci AE. 2012. The prevalence of pulp stones on a Turkish population. A radiographic survey. Med Oral Patol Oral 17:212–17. https://doi.org/10.4317/medoral.17400
Stipisic A, Versic-Bratincevic M, Knezovic Z, Sutlovic D. 2014. Metal content in medieval skeletal remains from Southern Croatia. J Archaeol Sci 46:393–400. https://doi.org/10.1016/j.jas.2014.03.032
Tomczyk J, Komarnitki J, Zalewska M, Wiśniewska E, Szopiński K, Olczak-Kowalczyk D. 2014. The Prevalence of pulp stones in historical populations from the Middle Euphrates Valley (Syria). Am J Phys Anthropol 153:103–15. https://doi.org/10.1002/ajpa.22414
Tomczyk J, Turska-Szybka A, Zalewska M, Olczak-Kowalczyk D. 2017. Pulp stones prevalence in a historical sample from Radom, Poland (AD 1791–1811). Int J Osteoarchaeol 27:563–72. https://doi.org/10.1002/oa.2579
Turkal M, Tan E, Uzgur R, Hamidi MM, Çolak H, Uzgur Z. 2013. Incidence and distribution of pulp stones found in radiographic dental examination of adult Turkish dental patients. Ann Med Health Sci Res 3:572–76. https://doi.org/10.4103/2141-9248.122115
Final information
Acknowledgments
The research has been done using JEOL JSM5410 Scanning Electron Microscope in the Department of Cell Biology and Imaging, Institute of Zoology and Biomedical Research, Jagiellonian University. The maintenance of this equipment has been supported by a grant of the Faculty of Biology under the Strategic Programme Excellence Initiative at Jagiellonian University PSP: UIU/W18/NO/04.
Conflict of interest
Lead author Jacek Tomczyk is the President of the Polish Anthropological Society (Polskie Towarzystwo Antropologiczne) of which Anthropological Review is a flagship journal. He was not involved in the editorial handling of this article.
Author contributions
JT – research concept and design, critical revision of the article; PR – writing the article, final approval of the article; KG – collection and/or assemble of data, final approval of the article; GT – data analysis and interpretation, writing the article, final approval of the article.
Financial disclosure
This work was supported by the National Science Centre (Poland) between 2013 and 2017 (Grant No. 2013/11/B/HS3/04117).
Ethics statement
The human remains are curated at the Institute of Biological Sciences, Cardinal Stefan Wyszynski University. Research on the presented material did not require ethics committee approval.
Corresponding author
Jacek Tomczyk, Institute of Biological Sciences, Cardinal Stefan Wyszyński University, Wóycickiego 1/3 St., 01-938 Warsaw, Poland; e-mail: j.tomczyk@uksw.edu.pl