Eruption of Permanent Teeth in Bulgarian Children Aged 5–12 Years
Boyan Valentinov Kirilov
 https://orcid.org/0000-0002-6954-7296
https://orcid.org/0000-0002-6954-7296
Department of Anthropology and Anatomy, Institute of Experimental Morphology, Pathology and Anthropology with Museum, Bulgarian Academy of Sciences, Sofia, Bulgaria
Ivaila Yankova Ivanova-Pandourska
 https://orcid.org/0009-0001-6637-705X
https://orcid.org/0009-0001-6637-705X
Department of Anthropology and Anatomy, Institute of Experimental Morphology, Pathology and Anthropology with Museum, Bulgarian Academy of Sciences, Sofia, Bulgaria
Yanitsa Velichkova Zhecheva
 https://orcid.org/0000-0002-6028-7440
https://orcid.org/0000-0002-6028-7440
Department of Anthropology and Anatomy, Institute of Experimental Morphology, Pathology and Anthropology with Museum, Bulgarian Academy of Sciences, Sofia, Bulgaria
Abstract. The time of eruption and the number of permanent teeth together with the time of sexual maturation and ossification of the skeleton are important indicators of the biological maturity and health of children. The aim is to evaluate the eruption of permanent teeth in Bulgarian children aged 5–12 years and to assess its relationship with age and sex. The present cross-sectional study included 709 individuals from 5 to 12 years. The oral and dental status was checked and included the number of erupted teeth (NET). Statistical analyses were performed to compare the sexes and ages. Statistically significant differences between sexes are observed only in teeth 17, 27, 33 and 43. The sequence of tooth eruption was examined, and it is almost identical in male and female subadults. Mandibular teeth erupt earlier than maxillary teeth, excluding first premolars for both sexes and canines in males. Females have earlier tooth eruption and more permanent teeth compared to males. A significant association between age and NET is established. Sex does not have a significant effect on tooth eruption. The time of eruption of permanent teeth is influenced significantly by age, but not by sex in our sample. Differences between males and females are found in the sequence of eruption only of canines and premolars. Females tend to have earlier tooth eruption and more erupted teeth. Lower teeth erupt earlier than upper teeth, excluding first premolars in both sexes and canines in males.
Keywords: children, tooth eruption, sequence, sex differences, age differences
Introduction
The time of eruption and the number of permanent teeth (NET) together with the time of sexual maturation and ossification of the skeleton are important indicators of the biological maturity and health of children (Nikolova et al. 2023). Tooth eruption is a long physiological process during which the forming tooth penetrates the oral mucosa, and the incisal edge or tip of a cusp is seen in the oral cavity (Peneva et al. 2009:141–2). As an indicator of the development of the maxillofacial complex, tooth eruption is used in dentistry to evaluate disturbances in the process of eruption and to determine measures and prepare plans for treatment and optimize children’s oral health (Leroy et al. 2003; Wright 2010; Kutesa et al. 2013).
In forensic odontology and paleoanthropology, the formation and eruption of teeth are used to identify human remains and to estimate age and diet of individuals. Factors which are used for determination of dental age are degrees of formation of unerupted teeth, clinical eruption, degree of completion of roots of erupted teeth, degree of resorption of deciduous teeth, which are all part of the process of tooth eruption (Jayakrishnan et al. 2021). Several anthropological studies have shown that the time of eruption of permanent teeth varies between different populations. A lot of factors influence this process, including genetics, hormones, age, sex, ethnicity, socioeconomic status, nutrition, craniofacial morphology, pathology (e.g., Almonaitiene et al. 2010; Kutesa et al. 2013; Kurosaka et al. 2022:159–164).
Eruption of permanent teeth follows a specific sequence, and it is related to the age of the individual. It is clinically observed at the age of 6 and ends around 12 years of age for permanent teeth (Peneva et al. 2009; Paz-Cortés et al. 2022). In Bulgaria, there are few studies investigating the time and sequence of dental eruption and its relationships with different factors (Peneva 1978; Mladenova 2003; Tineshev 2009). The importance of the present study is to provide new data on the mean age and sequence of eruption of permanent teeth in Bulgarian children. The aim is to evaluate the eruption of permanent teeth in Bulgarian children aged 5–12 years and to assess its relationship with age and sex.
Materials and methods
A total of 709 children from 4 kindergartens and 11 schools, were investigated during the period 2020–2023 by the first author. This study received ethics approval by the Ethical Committee of the Institute of Experimental Morphology, Pathology and Anthropology with Museum – Bulgarian Academy of Sciences (Protocol 10/08.07.2020). Before starting the examination, information about the purpose of the study was given and written informed consent was obtained. The study was conducted in accordance with the principles of the Declaration of Helsinki for human studies (World Medical Association 2008) (see details in the Ethics Statement below). The individuals/participants were randomly selected according to the following criteria:
- Inclusion criteria: Children aged 5–12 years in good physical and psychological health; informed written consent given by parents or guardians.
- Exclusion criteria: children with systemic and chronic diseases and lack of age data.
The examination was performed in the medical rooms in the schools and kindergartens. The oral and dental status was checked directly by intraoral examination with a dental mirror and a probe under artificial light. Permanent teeth were categorized into two grades: 1 – erupted and 0 – unerupted, and all erupted teeth were assessed for each child. The tooth was registered as erupted when any part of the clinical crown was present inside the oral cavity. The number of erupted permanent teeth was evaluated for the studied age groups.
Data were analyzed using Statistical Package for Social Sciences (SPSS Inc, version 16.0 for Windows, IBM Corp Chicago, IL, USA). Pearson Chi-square test, Cramer’s V and Odds ratio were used to study the association between age, sex and eruption of permanent teeth. Binary Logistic Regression also was performed. The Student’s t-test and One-Way ANOVA (with post hoc Scheffe multiple comparison procedures) were applied to estimate differences between sexes and age groups. The differences were considered statistically significant at p<0.05.
Results
The present cross-sectional study included 361 (50.9 %) male and 348 (49.1 %) female subadults. The examined participants did not differ significantly in mean age (males – 8.79 (± 2.20) and females – 8.68 (± 2.20) years, p>0.05 ). The average age of tooth eruption is presented on Table 1. Statistically significant differences between sexes are observed only in teeth 17, 27, 33 and 43 /upper second molars and lower canines/, which erupted earlier in females (p < 0.05).
| Males | no data | Females | Т-test (p value) ♂/♀ |
||
|---|---|---|---|---|---|
| Maxilla | no data | ||||
| Right (I) | Left (II) | Tooth | Right (I) | Left (II) | no data |
| 6.53 | 6.67 | 1/I1 | 6.64 | 6.64 | 17 – .039*; 27 – .023* |
| 7.38 | 7.49 | 2/ I2 | 7.34 | 7.35 | |
| 10.18 | 10.26 | 3/ C | 10.36 | 10.45 | |
| 9.22 | 9.37 | 4/ P1 | 9.37 | 9.37 | |
| 10.29 | 10.38 | 5/ P2 | 10.32 | 10.27 | |
| 6.45 | 6.47 | 6/ M1 | 6.50 | 6.48 | |
| 12.09 | 12.20 | 7/ М2 | 11.89 | 11.84 | |
| Mandibula | no data | ||||
| Right (IV) | Left (III) | Tooth | Right (IV) | Left (III) | no data |
| 6.37 | 6.36 | 1/I1 | 6.27 | 6.28 | 33 – .027*; 43 – .004* |
| 7.38 | 7.36 | 2/ I2 | 7.30 | 7.29 | |
| 10.24 | 10.39 | 3/ C | 10.15 | 10.11 | |
| 9.31 | 9.44 | 4/ P1 | 9.38 | 9.38 | |
| 10.14 | 10.22 | 5/ P2 | 10.30 | 10.32 | |
| 6.40 | 6.38 | 6/ M1 | 6.42 | 6.37 | |
| 11.87 | 11.82 | 7/ М2 | 11.76 | 11.73 | |
Level of significance: * p<0.05
The sequence of tooth eruption is almost identical in male and female individuals and is as follows:
In males: I1, М1, М1, I1, I2, I2, P1, P1, P2, C1, C1, P2, M2, M2
In females: I1, М1, М1, I1, I2, I2, P1, P1, C1, P2, P2, C1, M2, M2
Mandibular teeth erupt earlier than the maxillary teeth, excluding first premolars for both sexes and canines in males. Differences are observed only in the eruption of the premolars and canines. In males, teeth 35 and 45 erupt before the canines followed by upper premolars, while in females, teeth 33 and 43 erupt before the premolars followed by upper canines.
During the studied age period females have earlier tooth eruption and more permanent teeth compared to males. The mean number of erupted teeth varied between 1.36 (± 1.27) at the age of 5 and 26.03 (± 2.41) at the age of 12. In males the mean number of erupted teeth is lower and varied between 1.84 (± 2.84) and 25.60 (± 3.4). A significantly higher NET in females is observed in the age groups 7, 10 and 11 years (Fig. 1).
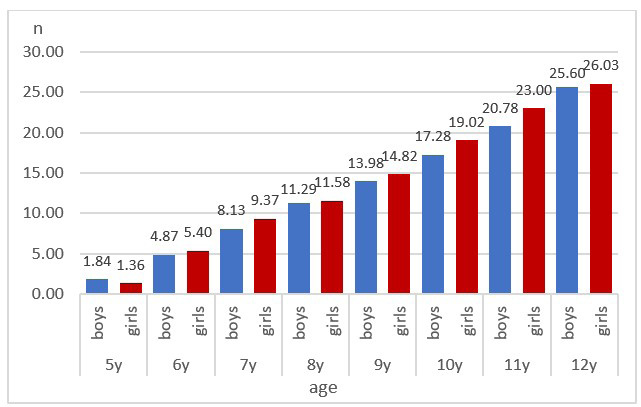
A significant association between age and NET is established (χ2(7, N = 709) = 352.08, p < 0.0001, Cramer’s V = 0.705,). This is the reason to consider that age is an important factor in tooth eruption. The results from logistic regression model show that older age has a significant role in tooth eruption (OR=14.420, p < 0.0001, 95 % CI (7.583–27.422)). For every year increase in age the odds are 14.4 times larger.
Unlike age, sex does not significantly affect tooth eruption (χ2(1, N = 709) = 0.039, p = 0.843, Cramer’s V = 0.007). The null hypothesis that the two variables are independent of each other cannot be rejected. The females and males seem to have nearly equal odds (OR = 1.05) of erupted teeth (Table 2).
| no data | no data | no data | Permanent teeth | Total | |
|---|---|---|---|---|---|
| Unerupted | Erupted | ||||
| Sex | Males | n | 39 | 322 | 361 |
| % | 10.8% | 89.2% | 100.0% | ||
| Females | n | 36 | 312 | 348 | |
| % | 10.3% | 89.7% | 100.0% | ||
| Total | no data | n | 75 | 634 | 709 |
| % | 10.6% | 89.4% | 100.0% | ||
Discussion
Tooth eruption is an essential part of children’s growth and development. It is genetically determined and X chromosome linked inheritance. Reduplication in the X chromosome in females can be associate with higher intra individual correlations between factors of tooth eruption (Garn et al. 1965).
Some authors have established earlier tooth eruption and more erupted teeth in females at ages 6 to 14 in comparison to males (Amidu et al. 2013; Sheetal et al. 2013:178–80; Öznurhan et al. 2016; Hassan and Shahid 2018). In our study the average age of tooth eruption is almost identical for both sexes, but eruption in females started earlier than in males. Our data show that the first teeth to erupt in both sexes are the lower central incisors (31, 41). The biggest difference, between the two sexes is found in canines – 4 to 6 months earlier eruption in females. Bayrak et al. (2012) present results that show difference in the eruption times between males and females up to 18 months. In the study conducted by Wedl (2004) the eruption times of the incisors in both sexes are lower compared to our results, but all other examined teeth have higher mean age of eruption. Kapusi-Papp et al. (2023) also found statistically significant earlier mean eruption times for all teeth in females than in males, except for the mandibular central incisors. The difference varies from 1.9 up to 8.9 months. According to Eskeli et al. (2016) and Šindelářová et al. (2018) there were significant differences in the eruption status between both sexes in Finland and Czech populations, respectively. Rehmawati et al. (2022) established differences between males and females only in the eruption of teeth 16, 26, 36 and 46. Earlier physical development in females is a factor that determines their earlier tooth eruption (Paz-Cortés et al. 2022). Expression of genes coded in the Y-chromosome is believed to be connected with delayed skeletal maturity and tooth eruption in males (Nassif and Sfeir 2020).
The first clinically visible stage of the process of eruption is the penetration through the oral mucosa of the tip of the dental crown and is associated with the formation of enamel and dentin layers (Kutesa et al. 2013).
Aris et al. (2020) examined daily secretion rates (DSRs) of enamel in anterior teeth in males and females from ancient and modern British populations. They did not find differences in enamel DSRs between male and female groups, but comparing the different British populations the authors observed significantly delayed enamel DSRs in the modern-day populations compared to ancient ones. Those results are leading to the conclusion that the process of forming the enamel is not determined by sex differences in tooth eruption and sequence of eruption. Aris (2022) also compared enamel DSRs in anterior and posterior teeth and did not find any trend in which teeth tend to grow faster.
The timing of tooth eruption varies among different populations. Baka pygmy individuals are found to have the earliest tooth eruption compared to other human populations (Ramirez Rozzi 2016). Olze et al. (2007) in their study compared eruption of wisdom teeth in individuals from different ethnicities. They found that black South African population are reaching eruption stages faster than the other examined populations and Japanese are the slowest. Examining the mean eruption times in males and females aged 4–15 years from Uganda Kutesa et al. (2013) established that the values were comparable to Ghanaian and Nigerian children but lower than these of subadults from Belgium, USA, Australia, Iran and Pakistan.
Differences between sexes are also observed in the sequence of dental eruption. Females, more frequently than males, are found to have the most common sequence of eruption (Suri et al. 2004; Peneva et al. 2009; Arid et al. 2017; Reis et al. 2021; Kurosaka et al. 2022), in which lower teeth erupt earlier than the upper ones (Mladenova 2003; Amidu et al. 2013; Nikolova et al. 2023). Our results show that the eruption in both sexes starts with the lower central incisors, after them lower first molars erupt, upper first molars, upper central incisors, lower lateral incisors, upper lateral incisors, upper first premolar, lower fist premolar. Difference is observed in the sequence of eruption of second premolars and canines – for males the order of eruption is: lower second premolars, upper canines, lower canines, upper second premolars and for females – lower canines, lower second premolars, upper second premolars and upper canines. After that, tooth eruption is similar for both sexes and continues with lower second molars and upper second premolars. For both females and males is valid eruption firstly of the lower teeth and after them the upper, with the exclusion of first premolars for both sexes and canines in males which erupt firstly in upper jaw.
Our results contrast with the studies by Peneva (1978), Mladenova (2003) and Nikolova et al. (2023) on Bulgarian children. According to these authors the first teeth to erupt are the lower first molars followed by the incisors. Other differences are established in the eruption of the premolars and canines. The sequence described by Peneva (1978) for males is: lower first premolars, lower canines, upper canines, upper second premolars, lower second premolars and for females it is: lower canines, lower first premolars, upper second premolars, upper canines, lower second premolars, which quite differs from our results. Earlier tooth eruption in the lower jaw, excluding premolars, is found by Peneva (1978). This conclusion is based on the earlier development of lower tooth germs and the more active mandible in the process of mastication. Our results for the sequence of eruption of the permanent teeth are similar to results presented by Oznurhan et al (2016) – the difference is in upper canines, which erupt later than second premolar in males and lower canines which erupt earlier than the first and second premolar. Almost identical difference is found in the study done by Nassif and Sfeir (2020). In their study difference between the sexes is not presented and again upper canines erupt later than premolars and lower canines. A similar sequence is found in the study made by Bayrak et al. (2012) and Kapusi-Papp et al. (2023) who reported that the lower canine erupts earlier than the lower premolar. In their study, no difference is established between the sexes in eruption of permanent teeth of lower jaw.
Conclusion
The time of eruption of permanent teeth is influenced significantly by age, but not by sex. Differences between males and females are found in the sequence of eruption only of canines and premolars. Females tend to have earlier tooth eruption and more erupted teeth during the age period 5–12 years. Lower teeth are erupting earlier than the upper, excluding first premolars for both sexes and canines in males.
The results present contemporary view of tooth eruption in Bulgarian children, which can be used from a variety of clinicians such as orthodontists, pediatric dentists, pediatricians and forensic pathologists.
References
Almonaitiene R, Balciuniene I, Tutkuviene J. 2010. Factors Influencing Permanent Teeth Eruption. Part one – general factors. Stomatologija, Baltic Dent Maxill J 12(3):67–72.
Amidu N, Owiredu W, Saaka M, Quaye L, Wanwan M, Kumibea P, Mogre V. 2013. Determinants of Childhood Obesity Among Basic School Children Aged 6 – 12 Years in Tamale Metropolis. J Medd Biomed Sci 2(3):26–34. https://doi.org/10.4314/jmbs.v2i3.5
Arid J, Vitiello MC, da Silva RAB, da Silva LB, Mussolino de Queiroz A, Küchler E, Nelson-Filho P. 2017. Nutritional Status is Associated with Permanent Tooth Eruption Chronology. Braz J Oral Sci 16:1–7. https://doi.org/10.20396/bjos.v16i0.8650503
Aris C. 2022. Enamel growth rate variation of inner, mid, and outer enamel regions between select permanent tooth types across five temporally distinct British samples. Arch Oral Biol 137, 105394. https://doi.org/10.1016/j.archoralbio.2022.105394
Aris C, Mahoney P, Deter C. 2020. Enamel growth rates of anterior teeth in males and females from modern and ancient British populations. Am J Phys Anthropol 173:236–249. https://doi.org/10.1002/ajpa.24068
Bayrak S, Sen Tunc E, Tuloglu N, Acikgoz A. 2012. Timing of permanent teeth eruption in Turkish children. J Clin Pediatr Dent 37(2):207–11. https://doi.org/10.17796/jcpd.37.2.8v072017534j0191
Eskeli R, Lösönen M, Ikävalko T, Myllykangas R, Lakka T, Laine-Alava MT. 2016. Secular trends affect timing of emergence of permanent teeth. Angle Orthod 86(1):53–8. https://doi.org/10.2319/121014-894.1
Garn SM, Lewis AB, Kerewsky RS. 1965. Genetic, Nutritional and Maturational Correlates of Dental Development. J Dent Res 44(SUPPL):228–42. https://doi.org/10.1177/00220345650440011901
Hassan S, Shahid H. 2018. Assessment of Eruption of Permanent Teeth According to Age and Its Relation with Body Mass Index in Local Population. J of Pak Dent Assoc 27(03):127–132. https://doi.org/10.25301/JPDA.273.127
Jayakrishnan JM, Reddy J, Vinod Kumar RB. 2021. Role of forensic odontology and anthropology in the identification of human remains. J Oral Maxill Pathol 25(3):543–547. https://doi.org/10.4103/jomfp.jomfp_81_21
Kapusi-Papp Z, Máth J, Nemes JÁ. 2023. Timing and sequence of permanent tooth emergence in Hungarian children. Research Square Platform LLC. https://doi.org/10.21203/rs.3.rs-3215333/v1
Kurosaka H, Itoh S, Morita C, Tsujimoto T, Murata Y, Inubushi T, Yamashiro T. 2022. Development of Dentition: From Initiation to Occlusion and Related Diseases. J Oral Biosci 64(2):159–164. https://doi.org/10.1016/j.job.2022.02.005
Kutesa A, Nkamba EM, Muwazi L, Buwembo W, Rwenyonyi CH. 2013. Weight, Height and Eruption Times of Permanent Teeth of Children Aged 4–15 Years in Kampala, Uganda. BMC Oral Health 13. https://doi.org/10.1186/1472-6831-13-15
Leroy R, Bogaerts K, Lesaffre E, Declerck D. 2003. The Emergence of Permanent Teeth in Flemish Children. Community Dent Oral Epidemiol 31(1):30–39. https://doi.org/10.1034/j.1600-0528.2003.00023.x
Mladenova S. 2003. Anthropological Characterization of Growth and Evolution of Children and Teenagers in Contemporary Age at the Smolyan Region. Summary of PhD thesis, Plovdiv University (in Bulgarian)
Nassif N, Sfeir E. 2020. Age and Sequence of Permanent Teeth Eruption in Lebanese Children. Sci World J 9238679. https://doi.org/10.1155/2020/9238679
Nikolova M, Mladenova S, Tineshev Sl. 2023. Sexual Maturity and Teeth Growth at Children and Teenagers. Population Research Plovdiv: Plovdiv University Press (in Bulgarian).
Olze A, van Niekerk P, Ishikawa T, Zhu BL, Schulz R, Maeda H, Schmeling A. 2007. Comparative study on the effect of ethnicity on wisdom tooth eruption. Int J Legal Med 121:445–448. https://doi.org/10.1007/s00414-007-0171-9
Öznurhan F, Sungurtekin E, Özalp Ş, Deveci C., Delilbaşi AE, Bani M, Öztaş N. 2016. Time and Sequence of Eruption of Permanent Teeth in Ankara, Turkey. Pediatr Dent J 26(1):1–7. https://doi.org/10.1016/j.pdj.2015.09.002
Paz-Cortés MM, Muñoz-Cano L, Diéguez-Pérez M. 2022. Evaluation of the Relationship between the BMI and the Sequence and Chronology of Eruption in Permanent Dentition in Spanish Population. Healthcare (Basel) 10(6):1046. https://doi.org/10.3390/healthcare10061046
Peneva MD. 1978. Eruption of Permanent Teeth PhD thesis. Sofia. Medical university of Sofia (in Bulgarian).
Peneva M, Kabaktchieva R, Rashkova M, Tsolova E. 2009. Oral Embryology, Histology and Biology 3rd edition. Sofia: Iztok-zapad (in Bulgarian).
Ramirez Rozzi F. 2016. Diversity in tooth eruption and life history in humans: illustration from a Pygmy population. Sci Rep 6,27405. https://doi.org/10.1038/srep27405
Rehmawati AD, Rahayu S, Medawati A, Alphianti LT, Nurushifa N, Ranasti W. 2022. Permanent Teeth Eruption Status in Growing-Age Children with Normal Nutritional Status Based on Gender. In: I Permana, E Rochmawati, editors. Proceedings of the International Conference on Sustainable Innovation on Health Sciences and Nursing (ICOSI-HSN 2022), AHSR 55: 285–293 Atlantis Press. https://doi.org/10.2991/978-94-6463-070-1_34
Reis CLB, Barbosa MCF, Henklein S, Madalena IR, de Lima D, Oliveira M, Küchler E, de Oliveira D. 2021. Nutritional status is associated with permanent tooth eruption in a group of Brazilian school children. Glob Pediatric Health. 8:1–6. https://doi.org/10.1177/2333794X211034088
Sheetal A, Hiremath VK, Patil AG, Sajjansetty S, Kumar SR. 2013. Malnutrition and its oral outcome – a review. J Clin Diagn Res 7(1):178–80. https://doi.org/10.7860/JCDR/2012/5104.2702
Šindelářová R, Soukup P, Broukal Z. 2018. The relationship of obesity to the timing of permanent tooth emergence in czech children. Acta Odontol Scand 76(3):220–225. https://doi.org/10.1080/00016357.2017.1403649
Suri L, Gagari E, Vastardis H. 2004. Delayed tooth eruption: pathogenesis, diagnosis, and treatment: a literature review. Am J Orthod Dentofacial Orthop 126(4):432–45. https://doi.org/10.1016/S088954060400530X
Tineshev Sl. 2009. Anthropological Characterization of Children and Teenagers. Summary of PhD thesis. Plovdiv. Plovdiv University (in Bulgarian)
Wedl JS, Danias S, Schmelzle R, Friedrich RE. 2004. Eruption Times of Permanent Teeth in Children and Young Adolescents in Athens (Greece). Clin Oral Investig 9(2):131–4. https://doi.org/10.1007/s00784-004-0295-y
World Medical Association. 2008. Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. WMJ 54(4):122–25.
Wright JT. 2010. Anatomy and Development of the Teeth. UpToDate. Available through: www.uptodate.com [Accessed 18 May 2025].
Final information
Acknowledgements
The authors would like to thank all participants and their parents who voluntarily contributed to the success of this study.
Conflict of interests
No conflict of interest is declared by the authors.
Ethics approval
The study was approved by the Ethical Committee of the Institute of Experimental Morphology, Pathology and Anthropology with Museum – Bulgarian Academy of Sciences (Protocol 10/08.07.2020). Before starting the examination, information about the purpose of the study was given and written informed consent was obtained. The study was conducted in accordance with the principles of the Declaration of Helsinki for human studies (World Medical Association 2008).
Author contributions statement
BK and IYP design the study; BK and IYP collected the data; BK, IYP, YZ oversaw the statistical analysis and interpretation; BK, IYP and YZ were the authors of the written content; All authors agree to be accountable for all aspects of the work.
Corresponding author
Boyan Valentinov Kirilov, Department of Anthropology and Anatomy, Institute of Experimental Morphology, Pathology and Anthropology with Museum, Bulgarian Academy of Sciences, Acad. Georgi Bonchev Str., Bl. 25, 1113 Sofia, Bulgaria, e-mail: drkirilov@yandex.com