Available online at: https://doi.org/10.18778/1898-6773.87.4.07
 https://orcid.org/0000-0002-4199-1184
https://orcid.org/0000-0002-4199-1184
Department of Public Health, Witelon Collegium State University, Legnica, Poland
 https://orcid.org/0000-0002-1425-682X
https://orcid.org/0000-0002-1425-682X
Department of Human Biological Development, Adam Mickiewicz University, Poznan, Poland
ABSTRACT: The demographic crisis in Europe is growing due to an increasing proportion of couples with fertility disorders. The purpose of this study was to examine the variability of ovarian reserve markers with age in women with premature ovarian insufficiency (POI) and polycystic ovary syndrome (PCOS) in relative to women with normal ovarian function. Two hormones were analyzed: anti-Müllerian hormone (AMH) and folliculotropic hormone (FSH). This study demonstrates that AMH is a valuable indicator of alterations in reproductive capacity. FSH is a standard marker of the hypothalamic-pituitary-ovarian axis.
We examined the reproductive status of 390 women aged 23–46 in three groups. Ovarian dysfunction was determined by medical diagnosis. The study includes women with PCOS (n=154), POI (n=40), and control group (n=196) with normal ovarian function (NOF). Blood samples were collected to measure AMH and FSH. We used multivariate logistic regression analysis to demonstrate the relationship between hormone levels and age in different age groups. ANOVA was used to analyze factors related to AMH and FSH concentrations. The results confirmed that women with POI had significantly lower AMH concentrations and higher FSH concentrations than women with normal ovarian function only in the group of women aged 36–46 years. There were no statistically significant differences in FSH levels in women with POI and NOF in the 23–30 and 31–35 age groups. AMH levels were higher in the PCOS group than in women with NOF in all age groups. FSH marker did not differ compared to the control group in women aged 23–30 and 36–46. The predictive value of AMH in the diagnosis of PCOS is significantly higher than the commonly used FSH. The results may contribute to earlier assessment of biological status to support reproductive chances in women with POI and PCOS.
KEY WORDS: AMH, FSH, reproduction, fertility, PCOS, POI
European reports indicate an increasing prevalence of infertility among couples of reproductive age (EPAF – European Policy Audit on Fertility 2024). The European Society of Human Reproduction and Embryology recommends setting up research projects to improve reproductive health. In this study, we investigated the variation of anti-Müllerian hormone (AMH) and folliculotropic hormone (FSH) in relation to age among women with normal and impaired ovarian function such as premature ovarian insufficiency (POI) and polycystic ovary syndrome (PCOS). Endocrine regulation of the sexual cycle determines the normal functioning of the reproductive system. The AMH level is a conservative indicator of reproductive potential and is therefore one of the most stable markers of ovarian reserve. AMH is an indicator for early diagnosis of ovarian dysfunctions such as POI and PCOS. In the current literature, there are still too few data related to AMH and FSH variability in women with POI and PCOS. In this study, we investigated the variability of hormones in women with ovarian dysfunction in relation to chronological age.
Differentiation of the reproductive system during organogenesis follows two different pathways depending on sex determination. The initiation of AMH transcription is strictly controlled in terms of location and timing. During embryogenesis, transcription factors regulate sex-determined pathways for the hermaphrodite gonads (Svingen and Koopman 2013). During the sixth week of organogenesis, Wolff ducts differentiate. Under the influence of maternal estrogen, paired Müller ducts are formed. In the 10th week, in the absence of the Y chromosome, hermaphrodite gonads have not initiated the male pathway program in the second trimester of pregnancy. Consequently, they begin differentiation into ovaries by activating the expression of Wnt4 and Rspo1 genes.
The Wolf ducts degenerate, and the Müller ducts differentiate into fallopian tubes, the uterus and the upper vagina. Activation of the secretion of gonadoliberin in the hypothalamus stimulates the activity of the pituitary gland which, via the gonadotropic hormones FSH and LH, initiates the formation of ovarian reserve. The presence of AMH is detectable at 24 weeks of gestation in the granulosa cells of preantral follicles (Kuiri-Hanninen et al. 2011). The pool of ovarian follicles formed during fetal development will constitute the ovarian reserve of a woman of reproductive age (Nelson et al. 2007; Kwee et al. 2008; Blińska and Hejmej 2013).
The reproductive period of women is determined by ovarian reserve. The hormones AMH and FSH regulate the recruitment of ovarian follicles during folliculogenesis (La Marca et al. 2009; McGee and Hsueh 2006). AMH is responsible for managing the pool of follicles during their recruitment to the preantral follicle stage. Further follicle maturation occurs under the control of FSH. During ovulation, an oocyte is released from the Graaf follicle, and the follicular phase transitions to the luteal phase of the menstrual cycle.
AMH is a dimeric glycoprotein composed of 560 amino acids. Due to the presence of a C-terminal domain, it belongs to the β-TGF superfamily of transforming growth factors (Cohen-Haguenauer et al. 1987; Knight and Glister 2006; La Marca et al. 2009). The gene encoding AMH is located on chromosome 19p13.3.
Activation of the precursor form of AMH determines binding to the AMHR-II receptor and activation of the signaling cascade in target cells through tyrosine phosphorylation and activation of SMAD signaling proteins (di Clemente et al. 2010).
The number of AMHR receptors and their location are variable and dependent on age. The AMH-receptor system and the control of the menstrual cycle by the hypothalamic-pituitary-ovarian (HPO) axis determine reproductive fitness from menarche to the depletion of ovarian reserve. With the loss of follicles, AMH levels decline and expire (Kerkhof et al. 2010; Hagen et al. 2010; Kelsey et al. 2011; Lee et al. 2012; Lie Fong et al. 2012).
FSH is produced by basophilic cells of the anterior pituitary lobe under the influence of increased gonadoliberin (GnRH) release in the hypothalamus. FSH in the presence of estrogen regulates the formation of receptors for FSH (FSH-R) (Macklon and Fauster 2011). FSH affects the granulosa layer, resulting in the growth and development of the ovarian follicle (Skałba 2008; Gardner and Shoback 2011). FSH also stimulates an increase in aromatase activity, which is essential in the conversion of androgens to estrogens (Grossman et al. 2008).
FSH with androgens and non-ovarian factors, controls the qualitative and quantitative recruitment and selection of antral follicles to the dominant follicle stage before ovulation. A reduction or absence of FSH results in a decrease in the number of maturing follicles (Dewailly et al. 2016, Kumar et al. 2018).
FSH is a glycoprotein consisting of two peptide chains α and ß. The α subunit (89 amino acids) is characteristic of other gonadotropins. The ß subunit (115 amino acids) defines the specificity of each of the gonadotropic hormones (Gardner and Shoback 2012). The gene encoding FSHß is located on chromosome 11p13. and can be subject to mutations that affect the process of steroidogenesis or follicle recruitment in the ovary.
AMH levels reflect the secretion of only those follicles that are vascularised. AMH has auto- and paracrine effects. Follicles with impaired vascularisation are subject to atresia. At 18 weeks gestation, there are approximately 7 million follicles in the fetal ovaries (de Velde and Pearson 2002). As a result of atresia, the pool of ovarian follicles is reduced to approximately 1–2 million at birth.
At the time of menarche, there are 400,000 follicles in the ovaries and only 1,000 at menopause. The age of onset of natural menopause varies by population and in Poland falls, on average, at 51.2 years of age (Kaczmarek 2007).
As the ratio of primary and primordial follicles to the rarer pre-antral and antral follicles change with age, AMH levels decline and a high percentage of follicles undergo degenerative processes. This results in a decrease in ovarian volume and physiological (menopause) or accelerated cessation of ovarian activity – POI (Urutia et al. 2019). In women with POI, AMH levels decreased with age in all analyzed age groups.
Endocrine and metabolic abnormalities related to the HPO axis may contribute to POI. Elevated FSH levels are a response to poor follicular development during folliculogenesis. Too low estrogen levels affect increased FSH secretion, causing a change in the ratio of LH to FSH, a decrease in the level of sex hormone-binding globulin, and consequently a disruption of ovulation or the occurrence of non-ovulatory cycles.
The negative impact of both endogenous and exogenous factors has been identified as a potential cause of PCOS. This endocrinopathy, which is most commonly diagnosed among women of reproductive age, is characterized by high AMH and androgen levels. The excess hormones block the passage of follicles to the next stage of development. There is an accumulation at the preantral and early antral stages (Carlsen et al. 2009; Cessar et al. 2014; Pigny et al. 2003; Piltonen et al. 2005; Eldar-Geva et al. 2005; Homburg et al. 2013; Tal et al. 2014). In patients with PCOS, an increase in granulosa layer mass was observed in the granulosa cells of ovarian follicles. Increased AMH release by granulosa cells affects the cells of the inner follicle sheath, disrupting the conversion of androgens to estrogens. This causes hormonal imbalance and promotes the onset of PCOS symptoms (Ingraham et al. 2000). One of them is the difficulty of achieving pregnancy.
A total of 390 women were included in the study after giving voluntary and written consent to participate. The study was conducted in the period 15.07.2015 – 31.06.2016. The study group consisted of women aged 18–46 years without general medical treatment for at least one month prior to participation in the present study.
Accepted exclusion criteria are pregnancy and breastfeeding, cancer, endocrine disease, hormone therapy, hormonal contraception, removed uterus or ovary, thyroid disorders, and abnormal prolactin levels.
Based on clinical diagnosis, women were allocated to three groups: PCOS (n=154), POI (n=40), and a group with NOF (n=196). The control group consisted of women who had no abnormalities in reproductive function and came to the clinic because of problems occurring on their partner’s side. The group was divided into three age cohorts: 23–30, 31–35, and 36–46 years. Serum AMH and FSH levels were determined using the ECLIA immunoassay method in the laboratories of the Department of Infertility and Reproductive Endocrinology of the GPSK UM in Poznan and the InviMed European Motherhood Centre in Wrocław. Three categories of ovarian function were determined in the study: NOF, POI, and PCOS, and an indicator was adopted – serum AMH levels in the reference range for NOF women: 1.0–3.2 [ng/ml], POI < 1.0 [ng/ml] and PCOS > 3.2 [ng/ml]. The reference values for hormones were established in accordance with the prevailing laboratory guidelines at the time of the study. The study was conducted in a clinical department with the approval of the director of the Department of Gynaecology, Obstetrics, and Gynaecological Oncology, University of Medical Sciences, Poznan and accepted by the local Bioethics Committee.
The object of quantitative analysis is blood serum. For the determination of AMH and FSH profiles, a single blood sample was taken under ambulatory conditions. Due to the variability in the menstrual cycle, FSH was determined from a blood sample taken in the morning, fasting, on days 2–3 of the menstrual cycle.
The data obtained from the surveys were subjected to statistical analysis in the program package STATISTICA 13.3 statistics (StatSoft, Inc., 2014). The number of categories for each factor was determined by the value n, which corresponds to the frequency of hormone determinations of the hormone study. A significance level of p < 0.05 was used. Sociodemographic status data and their number and percentage were included in the basic descriptive statistics. Relationships between categorical variables were compared using the χ2 test. The quantitative variables included in the groups were described by mean ± standard deviation (SD). ANOVA test was used to assess differences between groups. The effect size was evaluated using partial eta squared (η2) and classified as: no effect = 0 to 0.039, minimum effect = 0.04 to 0.24, moderate effect = 0.25 to 0.63, and strong effect = ≥ 0.64. Regression and r-Pearson correlation analysis were used to assess the degree of association between hormone concentrations and modulating factors. The mean age of women with POI= 35.9 (±4.4) years, with PCOS=33.2 (±4.2). Of the 461 women, 71 did not consent to participate in the study or were over the age of 46 years. Mean baseline AMH levels in women with POI and PCOS were 0.56 (±0.3) ng/ml and 6.84 (±6.08) ng/ml, respectively. Mean FSH levels on cycle day three were 3.62 (±11.30) mlU/ml for POI and 6.04 (±1.79) mlU/ml for PCOS. Women were divided into three age cohorts: 23–30, 31–35, and 36–46 years. Most women with POI (60%) were aged 36–46 years with mean values of AMH 0.49 (±0.32) ng/ml, FSH taken on day three of menstrual cycle 10.96 (±14.42) mlU/ml. Most women with PCOS (44.81%) within the 31–35 age range exhibited AMH levels. The study revealed that most women with PCOS (44.81%) within the 31–35 age range exhibited AMH levels of 6.76 ng/ml (±4.04), FSH 10.96 mlU/ml (±1.61). In women with PCOS, AMH levels demonstrated a statistically significant increase with age, reaching a peak in the 36–46 years age group. The mean FSH value (5,94±1,65 – 6,10±1,61) in women with PCOS remained consistent across all age groups, demonstrating no correlation with age.
To trace the changes associated with depletion of ovarian reserve in women with normally functioning ovaries, the study group was divided into three age ranges: 23–30, 30–35, and 36–46 years. The division adopted provided for the comparison of parameters describing the condition of ovaries with POI and PCOS with the NOF group and to assess changes with age. Among women with PCOS, the largest group was between 31 and 35 years (45%). The number of youngest and oldest women was identical (27,5%).
The smallest number of women with NOF was in the 23–30 years group (18.4%). The ranges 31–35 (40.3%) and 36–46 (41.3%) years had similar numbers. Due to the accelerated loss of ovarian follicles in women with POI, the age of menopause will shift and occur earlier than the median age of physiological menopause in Poland of 51.25 years (Kaczmarek 2007).
The result of comparing the mean AMH concentration values in the impaired (POI, PCOS) and normal (NOF) groups indicated a significant difference between the groups (p< 0.01). A similar result was obtained when comparing the mean values of FSH concentrations in the study groups (p<0.01).
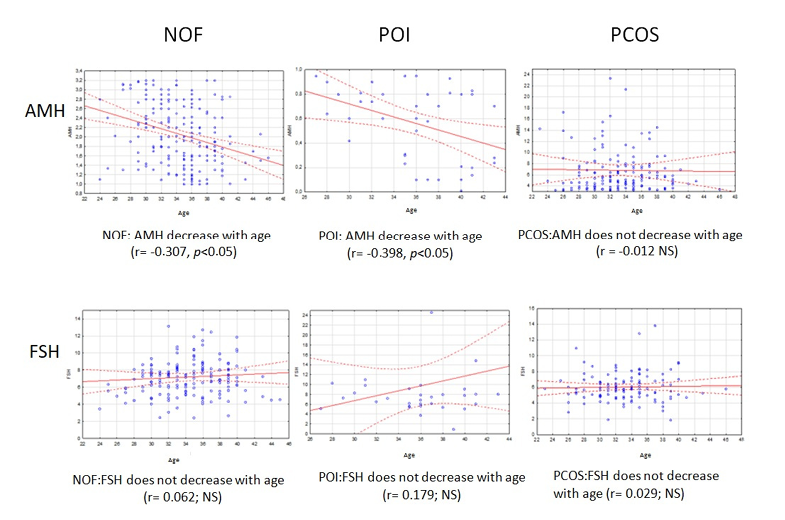
The regression analysis performed to determine the age-dependent variability of AMH and FSH concentrations revealed the differences between mean hormone concentrations in subjects with POI and PCOS compared to the NOF group in all age categories (Fig. 1). Results showed a negative correlation between age and AMH levels in the NOF group (r = -0.31, p < 0.05). A similar results was observed in women with POI, where the decline in hormone levels with age was even more evident (r = -0.40, p < 0.05). In the PCOS group, there was no significant association between mean AMH levels and age (r = -0.01; p>0.05), where the concentration of the hormone under study remained stable.

Figure 1. Variability of AMH and FSH levels among women with NOF, POI and PCOS
*NOF-normal ovary function; POI-premature ovarian insufficiency; PCOS-policistic ovarian syndrome; NOF: normal ovarian function
Regression analysis indicated a lack of association between FSH concentrations and age in both women in the ovarian dysfunction groups: POI (r=0.18; p>0.05) and PCOS (r=0.03; p>0.05), as well as control (r = 0.06; p>0.05). While not statistically significant, the regression model for the POI group indicates that there is a tendency for FSH levels to increase with age. In women with NOF, there was a statistically significant inverse correlation between age and AMH concentrations (p<0.01), which is consistent with the physiological norm. Conversely, the analysis demonstrated that FSH concentrations remained stable across all age ranges (p>0.05).
In the POI group, FSH levels also did not change with age (p>0.05), while the results showed a negative correlation in relation to AMH levels and age (p<0.05). Furthermore, it was observed that the oldest group of women (60%) had critically depleted ovarian reserve compared to the youngest and middle group. A comparison of mean AMH levels showed that in the 23–30 years group, POI women had lower AMH levels than NFO women at the same age (0.72±0.20 vs. 2.42±0.69g/ml).
However, there was no statistical significance (p>0.05) in the analysis of mean AMH levels between women in the POI 31–35 years group and the NFO group. Also, the comparison of predictor levels between POI and NFO in the 36–46 age range did not show statistical significance (p>0.05), although a clearer downward trend was observed in POI women.
AMH analysis in a group of women with POI in three age ranges: 23–30, 31–35 and 36–46 years showed that serum AMH levels observed in the youngest group of women were approximately threefold lower than those observed in the NOF group.
A comparable outcome was observed in the 31–35 age group, wherein the mean AMH concentration among women with POI was also threefold lower in comparison to the control group. The observation in the oldest group confirmed that the mean hormone level in women with POI aged 36–46 years was already four times lower compared to women with NFO of the same age. The linear decrease in AMH levels in POI women relative to NFO women with age was observed.
A certain limitation of the variable testing was the slightly lower number of POI women compared to the other groups, however, despite not reaching statistical significance (p>0.05), the observation of a trend was as expected.
The observation of AMH variability with age is confirmed by the negative correlation with FSH in the age ranges analyzed.
The analysis of mean FSH levels in women between the ages of 36 and 46 with POI confirms the elevated mean concentration in the NOF group. The mean FSH level is 1.5 times higher than that observed in the control group (p<0.05). There was no significant difference (p>0.05) in FSH levels when comparing the POI and NOF groups of women aged 31–36 years and the youngest group.
In women with PCOS, no correlation was identified between AMH concentration and age across all age categories examined (p>0.05). A similar result was obtained in the analysis of FSH, which indicated that there was no relationship between the concentration of this hormone and age (p>0.05) in women with PCOS. The obtained values of mean AMH and FSH levels in the three age groups according to the ovarian function status of POI, PCOS, and NOF are presented in Table 1.
Analysis of hormone concentrations in the NOF women group for each age category showed that AMH levels assumed the highest value in the 23–30 years group and decreased linearly with age. In contrast, FSH levels were higher in women aged 31–35 years compared to the younger group and then decreased slightly in the NOF 36–46 years group. AMH levels in women with POI, as in the control group, successively decreased with age, reaching the lowest level in the oldest group of subjects. The concentration of FSH in the group of women with POI showed some fluctuations, as its value decreased in the middle age range and then increased sharply in the 36–46 age range.
| Age | POI x ± SD |
NOF x ± SD |
PCOS x ± SD |
P-Value | η2 | |
| AMH [ng/ml] | 23–30 | 0.718 ± 0.20 | 2.425 ± 0.69 | 6.182 ± 3.49 | NSa; <0.01b | 0.69 |
| 31–35 | 0.638 ± 0.26 | 2.092 ± 0.62 | 6.762 ± 4.04 | NSa; <0.01b | 0.71 | |
| 36–46 | 0.491 ± 0.32 | 1.855 ± 0.59 | 7.465 ± 9.81 | NSa; <0.01b | 0.72 | |
| FSH [mlU/ml] | 23–30 | 7.690 ± 2.13 | 6.480 ± 1.57 | 5.944 ± 1.65 | NSa; NSb | – |
| 31–35 | 7.491 ± 1.98 | 7.510 ± 3.89 | 6.103 ± 1.61 | NSa; <0.01b | 0.41 | |
| 36–34 | 10.965 ± 14.42 | 7.223 ± 2.12 | 6.000 ± 2.27 | <005 a; NSb | 0.33 | |
Date expressed as mean ± standard deviation. p-Value was considered statistically significant. AMH: anti-Müllerian hormone, FSH: follicle stimulating hormone, POI: premature ovarian insufficiency, PCOS: PCOS-polycystic ovarian syndrome; NOF: normal ovarian function; a Comparison between POI and NOF, b Comparison between PCOS and NOF; NS: nonsignificantly different.
There was a significant difference in the comparison of AMH concentrations in women with PCOS and NOF according to age. Analysis of mean AMH concentration values in the NOF group by age range showed a tendency towards a physiological decrease in serum levels of the hormone, which was not confirmed in women with PCOS in the three age groups, where AMH values were increasing with age reaching the highest value in the oldest women (p<0.01). In the PCOS group, FSH levels remained constant in all age categories (5.94±1.65 – 6.10±1.61 mlU/ml) and did not differ with respect to mean FSH values in PCOS women in the youngest and oldest groups. Analysis showed a significant difference in FSH levels only in the 31–35 years category when comparing PCOS and NOF women (p<0.01).
The results of this study show that the proportion of women with PCOS is increasing and POI is observed in increasingly younger patients. The observed trend of late motherhood is increasingly unsuccessful due to the depletion of the ovarian reserve. The pool of ovarian follicles formed during prenatal development represents the reproductive potential of a woman of reproductive age until completely exhausted.
A growing number of studies point to the high predictive value of AMH and its role in the management of ovarian follicle resources (Lambert-Messalian et al 2016; Pankhurst et al. 2017; Pankhurst et al. 2016; Pankhurst 2019; Urrutia et al. 2019; Sova et al. 2019).
Early preventive diagnosis is an essential tool for monitoring reproductive health. In the present study, the concentrations of the ovarian reserve markers AMH and FSH were assessed in women with POI and PCOS compared to the NOF group over age. The analysis in this study showed that the age of the patients is the primary determinant affecting reproductive potential. Progressive involution of the gonads and a decrease in their endocrine activity results in impaired functioning of the hypothalamic-pituitary-ovarian axis (Kaczmarek and Wolanski 2018, Lambalk et al. 2009).
Changes in AMH and FSH concentrations indicate an increase in the rate of ovarian follicle loss by reversing the ratio of growing to primordial follicles. The present study showed that AMH concentrations in women with NFO in the three age ranges 23–30,31–35 and 36–46 years differed significantly, although these findings were not supported by the FSH levels, where the expected upward trend in the oldest women was not observed. The results obtained from the AMH analysis were in line with the findings reported in other studies (Bragg et al. 2012, Pankhurst et al. 2016).
Studies still under-represent results with the age category, which determines physiological norms for AMH and other sex hormones (Hambridge et al. 2013).
The regression analysis presented in this paper indicates that there is a linear decrease in AMH levels with age, while FSH fluctuations are less pronounced, particularly in women with ovarian dysfunction (Kumar et al. 2010; Randolph et al. 2014).
Similar results were reported by Visser and Themmen (2014) where the high predictive value of AMH for ovarian reserve was confirmed. A study by Pawelczyk and team (2003) showed that age determines the increased risk of reproductive disorders, such as POI and PCOS. The AMH regression analysis conducted in this study showed that POI women 23–30 years of age had significantly lower ovarian reserve than the NOF group. The differences in AMH levels in POI women, compared to the control group in subsequent age ranges, was statistically nonsignificant, albeit marked. Pankhurst (2017) conceptualized the role of AMH-mediated regulatory mechanisms in the primary activation of ovarian follicles to preserve fertility for as long as possible. According to the researcher, women with high ovarian reserve have efficient mechanisms to inhibit follicle loss in the ovaries. In women with POI, low concentrations of AMH accelerate the recruitment of primary ovarian follicles, which improves reproductive fitness but over a relatively shorter period of time compared to women with normal ovarian function. This is the cost of maintaining fertility in women with POI. The concept presented by Pankhurst and team is supported by the results of the present study. The reduction in AMH levels and the decline in concentration with age indicate that the reproductive capacity period in POI will be shorter than that observed in women with NFO.
The results obtained from the analysis of AMH levels in women with POI are consistent with the study by Urrutia et al. (2019).
In women’s POI, the relatively limited but fruitful reproductive period is followed by the cessation of ovarian function and the onset of premature menopause. AMH is the main factor modulating the process of inhibition in women of NOF or acceleration of follicle recruitment in the case of POI (Pankhrust 2017). The observed strategy promotes the optimization of the management of reproductive potential during a woman’s reproductive period.
Further research is needed to clarify the relationship between AMH symptoms and PCOS. Hormonal stability in the ovarian follicle is determined by a number of factors related to the regulation of trophic transitions such as FSH. In order to more accurately examine ovarian activity in the present study, in addition to the AMH marker, a second indicator standardly used to assess ovarian activity – FSH. It remains negatively associated with AMH (p<0.05) (Pigny et al. 2003) which supports findings reported by Sowers et al. (2010). The researchers conducted an analysis of ovarian reserve marker concentrations in 20 irregularly menstruating women between 20 and 30 years of age, which did not show a physiological pattern of ovarian aging consistent with chronological age.
The present study showed that the sensitivity of the standard marker FSH was significantly lower than AMH in women with PCOS. The analysis of AMH levels in the PCOS group showed a high diagnostic value in all age categories indicating a significant difference compared with the control group. The results obtained are in line with the results of other researchers who recommend AMH as a diagnostic tool for PCOS (Ray et al. 2012, Vale-Fernandes et al. 2023, Meczekalski et al. 2016, Liu et al. 2022). The comparison of mean FSH levels only in women 31–35 years with PCOS and PFJ showed a statistically significant difference which supports the higher sensitivity of AMH as an indicator of ovarian reserve. Sova et al. (2019) also showed a negative correlation between AMH and FSH levels in a group of women with PCOS (n=319) and healthy women (n=96) where the mean age was 28.1 years. FSH levels were not significantly different between the PCOS and control groups. The mean values of AMH levels indicated a difference at a high level of significance in both study groups (Sova et al. 2019). This result is consistent with the results obtained in this study, where the study group was included in three age categories due to the determining effect of chronological age on the physiological norm of AMH and FSH concentrations in NOF women.
This methodology permitted a more precise examination of the fluctuations in mean AMH and FSH levels in a cohort of women with impaired ovarian function, including those with POI and PCOS, while controlling for age. The results obtained by Grossman et al. (2008) indicated an increase in AMH levels in women with PCOS in the study group at the age of 28–37 years, which was also confirmed by the analyses in the present study. The survey conducted in this study had several limitations. The study focused on women from two regions of Poland, despite the large sample size, it is worth expanding the project to other regions of the country. The study included several variables affecting AMH and women’s reproductive status while future projects will require the inclusion of several other variables such as stress, lack of exercise, poor diet, overweight or diseases of the genitourinary system.
The findings of our study show that chronological age is a significant predictor of AMH levels and, as a result, of women’s reproductive potential. These findings suggest that AMH is more responsive to age-related changes than the conventional ovarian reserve marker FSH. Furthermore, the high predictive value of AMH for ovarian dysfunction was shown, particularly in the context of PCOS, where abnormalities were observed as early as age 23–30, while the FSH marker demonstrated insufficient sensitivity. Ovarian dysfunctions, such as POI and PCOS, can result in fertility issues. However, early diagnosis using anti-Müllerian hormone (AMH) testing can improve the likelihood of a successful pregnancy.
Acknowledgments
The authors would like to thank Prof. Leszek Pawelczyk and the staff of the Department of Infertility and Reproductive Endocrinology of GPSK UM, Karol Marcinkowski University of Medical Sciences in Poznan and management and staff of the InviMed European Motherhood Center in Wroclaw for their support and the opportunity to realize this work.
Conflict of interests
The authors declare no potential conflicts of interest regarding the research, authorship, or publication of this article.
Authors’ contribution
Both authors contributed equally to this manuscript.
Bragg JM, Kuzawa CW, Agustin SS, Banerjee MN, McDade TW. 2012. Age at menarche and parity are independently associated with anti-Müllerian hormone, a marker of ovarian reserve in Filipino young adult women. Am J Hum Biol 24:739–745. https://doi.org/10.1002/ajhb.22309
Carlsen S, Vanky E, Fleming R. 2009. Anti-Müllerian hormone concentrations in androgen-suppressed women with polycystic ovary syndrome. Hum Reprod 24(7):1732–1738. https://doi.org/10.1093/humrep/dep074
Cassar S, Teede HJ, Moran LJ, Joham AE, Harrison CL, Strauss BJ, Stepto NK. 2014. Polycysticovarysyndrome and anti-Müllerian hormone: role of insulinresistance, androgens, obesity and gonadotrophins. J Clin Endocrinol Metabol 81(6):899–906. https://doi.org/10.1111/cen.12557
Cohen-Haguenauer O, Picard JY, Mattéi MG, Serero S, Van Cong N, de Tand MF, et al. 1987. Mapping of the gene for anti-Müllerian hormone to the short arm of human chromosome 19. Cytogenet Cell Genet 44:2–6. https://doi.org/10.1159/000132332
Dewailly D, Robin G, Peigne M, Decanter C, Pigny P, Catteau-Jonard S. 2016. Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum Reprod Update 22(6):709–724. https://doi.org/10.1093/humupd/dmw027
Di Clemente N, Jamin SP, Lugovskoy A, Carmillo P, Ehrenfels C, Picard JY, et al. 2010. Processing of anti-Müllerian hormone regulates receptor activation by a mechanism distinct from TGF-β. Mol Endocrinol 24(11):2193–206. https://doi.org/10.1210/me.2010-0273
Eldar-Geva T, Margalioth EJ, Gal M, Ben-Chetrit, A, Algur N, Zylber-Haran E, Spitz IM. 2005. Serum anti-Müllerian hormone levels during controlled ovarian hyperstimulation in women with polycystic ovaries with and without hyperandrogenism. Hum Reprod 20(7):1814–1819. https://doi.org/10.1093/humrep/deh873
Gardner DG, Shoback D, Lewiński A. 2011. Endokrynología ogólna i kliniczna Greenspana, Tom.II. Wyd.2, Lublin: Wyd. Czelej.
Grossman MP, Nakajima ST, Fallat ME, Siow Y. 2008. Müllerian-inhibiting substance inhibits cytochrome P450 aromatase activity in human granulosa lutein cell Culture. Fertility and Sterility 89(5):1364–1370.
Guedikian AA, Lee AY, Grogan TR, Abbott DH, Largaespada K, Chazenbalk GD, Dumesic DA. 2018. Reproductive and metabolic determinants of granulosa cell dysfunction in normal-weight women with polycystic ovary syndrome. Fertility and Sterility 109(3):508–515. https://doi.org/10.1016/j.fertnstert.2017.11.017
Hagen CP, Vestergaard S, Juul A. 2012. Low concentration of circulating anti Müllerian hormone is not predictive of reduced fecundability in young healthy women: a prospective cohort study. Fertility and Sterility. 98:1602–1608. https://doi.org/10.1016/j.fertnstert.2012.08.008
Hambridge HL, Mumford SL, Mattison DR, Ye A, Pollack AZ, Bloom MS, Schisterman EF. 2013. The influence of sporadic anovulation on hormone levels in ovulatory cycles. Hum Reprod 28(6):1687–1694. https://doi.org/10.1093/humrep/det090
Homburg R, Ray A, Bhide P, Gudi A, Shah A, Timms P, Grayson K. 2013. The relationship of serum anti-Müllerian hormone with polycystic ovarian morphology and polycystic ovary syndrome: a prospective cohort study. Hum Reprod 28(4):1077–1083. https://doi.org/10.1093/humrep/det015
Kaczmarek M. 2007. The timing of natural menopause in Poland and associated factors. Maturitas 57(2):139–153. https://doi.org/10.1016/j.maturitas.2006.12.001
Kaczmarek M. 2007. Zróżnicowanie wieku menopauzy naturalnej wśród polskich kobiet ze względu na historie okresu rozrodczego. Przegląd Menopauzalny 2:69–76.
Kaczmarek M, Wolański N. 2018. Rozwój biologiczny człowieka od poczęcia do śmierci. Warszawa: 2018.
Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WH. 2011. A validated model of serum anti-Müllerian hormone from conception to menopause, PloS one 6(7):e22024. https://doi.org/10.1371/journal.pone.0022024
Kerkhof GF, Leunissen RW, Willemsen RH, de Jong FH, Visser JA, Laven JS, Hokken-Koelega AC. 2010. Influence of preterm birth and small birth size on serum anti-Mullerian hormone levels in young adult women. Eur J Endocrinol 163:937–944. https://doi.org/10.1530/eje-10-0528
Knight P, Glister C. 2006. TGF-β superfamily members and ovarian follicle development. Hum Reprod 132(2):191–206. https://doi.org/10.1530/rep.1.01074
Kuiri-Hänninen T, Kallio S, Seuri R, Tyrväinen E, Liakka A, Tapanainen J, Dunkel L. 2011. Postnatal developmental changes in the pituitary-ovarian axis in preterm and term infant girls. J Clin Endocrinol Metabol 96(11):3432–3439. https://doi.org/10.1210/jc.2011-1502
Kumar A, Kalra B, Patel A, McDavid L, Roudebush WE. 2010. Development of a second generation anti-Müllerian hormone (AMH) ELISA. J Immunol Methods 362(1–2):51–59. https://doi.org/10.1016/j.jim.2010.08.011
La Marca A, Broekmans FJ, Volpe A, Fauser BC, Macklon NS. 2009. Anti-Müllerian hormone (AMH): what do we still need to know? Hum Reprod 24(9):2264–2275. https://doi.org/10.1093/humrep/dep210
Lambalk CB, van Disseldorp J, De Koning CH, Broekmans FJ. 2009. Testing ovarian reserve to predict age at menopause. Maturitas 63(4):280–291. https://doi.org/10.1016/j.maturitas.2009.06.007
Lambert-Messerlian G, Plante B, Eklund EE, Raker C, Moore RG. 2016. Levels of antimüllerian hormone in serum during the normal menstrual cycle. Fertility and Sterility 105(1):208–213. https://doi.org/10.1016/j.fertnstert.2015.09.033
Lee JY, Jee BC, Lee JR, Kim CH, Park T, Yeon BR, et al. 2012. Age related distributions of anti-Müllerian hormone level and anti-Müllerian hormone models. Acta Obstetricia et Gynecologica Scandinavica 91:970–975. https://doi.org/10.1111/j.1600-0412.2012.01448.x
Lie Fong S, Visser JA, Welt CK, de Rijke YB, Eijkemans MJ, Broekmans FJ, Themmen AP. 2012. Serum anti-müllerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metabol 97(12):4650–4655. https://doi.org/10.1210/jc.2012-1440
Liu S, Hong L, Mo M, Xiao S, Wang X, Fan X, et al. 2022. Association of antimüllerian hormone with polycystic ovarian syndrome phenotypes and pregnancy outcomes of in vitro fertilization cycles with fresh embryo transfer. BMC Pregnancy Childbirth 22(1):171. https://doi.org/10.1186/s12884-022-04518-0
Macklon NS, Fauser BC. 2011. Follicle-stimulating hormone and advanced follicle development in the human. Arch Med Res 32:595–600. https://doi.org/10.1016/s0188-4409(01)00327-7
McGee EA, Hsueh AJ. 2000. Initial and cyclic recruitment of ovarian follicles. Endocrine Rev 21(2):200–214. https://doi.org/10.1210/edrv.21.2.0394
Meczekalski B, Czyzyk A, Kunicki M, Podfigurna-Stopa A, Plociennik L, Jakiel G, et al. 2016. Fertility in women of late reproductive age: the role of serum anti-Müllerian hormone (AMH) levels in its assessment. J Endocrinol Invest 39(11):1259–1265. https://doi.org/10.1007/s40618-016-0497-6
Nelson SM, Yates RW, Fleming R. 2007. Serum anti-Müllerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles—implications for individualization of therapy. Hum Reprod 22(9):2414–2421. https://doi.org/10.1093/humrep/dem204
Pankhurst MW, Clark CA, Zarek J, Laskin CA, McLennan IS. 2016. Changes in circulating proAMH and total AMH during healthy pregnancy and post-partum: a longitudinal study. PloSone 11(9):0162509. https://doi.org/10.1371/journal.pone.0162509
Pankhurst MW, Leathart BL, Batchelor NJ, McLennan IS. 2016. The anti-Müllerian hormone precursor (proAMH) is not converted to the receptor-competent form (AMHN, C) in the circulating blood of mice. Endocrinology. 157(4):1622–1629. https://doi.org/10.1210/en.2015-1834
Pankhurst MW. 2017. A putative role for anti-Müllerian hormone (AMH) in optimising ovarian reserve expenditure. J Endocrinol 233(1):R1–13. https://doi.org/10.1530/joe-16-0522
Pankhurst MW, Shorakae S, Rodgers RJ, Teede HJ, Moran LJ. 2017. Efficacy of predictive models for polycystic ovary syndrome using serum levels of two anti Müllerian hormone isoforms (proAMH and AMHN, C). Fertility and Sterility 108(5):851–857. https://doi.org/10.1016/j.fertnstert.2017.08.012
Pawelczyk L, Sokalska A, Serdyńska M. 2003. Płodność u kobiet w wieku przedmenopauzalnym. Przegląd Menopauzalny 1:14–18.
Pigny P, Merlen E, Robert Y, Cortet-Rudelli C, Decanter C, Jonard S, Dewailly D. 2003. Elevated serum level of anti-Müllerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metabol 88(12):5957–5962. https://doi.org/10.1210/jc.2003-030727
Piltonen T, Morin-Papunen L, Koivunen R., Perheentupa A, Ruokonen A, Tapanainen JS. 2005. Serum anti-Müllerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod 20(7):1820–1826. https://doi.org/10.1093/humrep/deh850
Randolph Jr, JF, Harlow SD, Helmuth ME, Zheng H, McConnell DS. 2014. Updated assays for inhibin B and AMH provide evidence for regular episodic secretion of inhibin B but not AMH in the follicular phase of the normal menstrual cycle. Human Hum Reprod 29(3):592–600. https://doi.org/10.1093/humrep/det447
Ray A, Shah A, Homburg R. 2012. Is there a role of antimullerian hormone in diagnosis of PCOS? a prospective cohort study. Fertility and Sterility 98(3):S53.1
Skałba P, Cygal A, Dąbkowska-Huć A. 2008. Wpływ hormonu anty-Müllerowskiego (AMH) na folikulogenezę. Ginekologia Polska 79:137–140.
Sova H , Unkila-Kallio L, Tiitinen A, Hippeläinen M, Perheentupa A, Tinkanen H. 2019. Hormone profiling, including anti-Müllerian hormone (AMH), for the diagnosis of polycystic ovary syndrome (PCOS) and characterization of PCOS phenotypes. Gynecol Endocrinol 35(7):595–600. https://doi.org/10.1080/09513590.2018.1559807
Sowers M, McConnell D, Gast K, Zheng H, Nan B, McCarthy JD, Randolph JF. 2010. Anti-Müllerian hormone and inhibin B variability during normal menstrual Cycles. Fertility and Sterility 94(4):1482–1486.
Stanisz A. 2007. Przystępny kurs statystyki z zastosowaniem STATISTICA PL na przykładach z medycyny. Kraków: StatSoft Polska.
Streuli I, Fraisse T, Chapron C, Bijaoui G, Bischof P, De Ziegler D. 2009. Clinical uses of anti-Müllerian hormone assays: pitfalls and promises. Fertility and Sterility 91(1):226–230. https://doi.org/10.1016/j.fertnstert.2007.10.067
Tal R, Seifer DB, Khanimov M, Malter HE, Grazi RV, Leader B. 2014. Characterization of women with elevated antimullerian hormone levels (AMH): correlation of AMH with polycystic ovarian syndrome phenotypes and assisted reproductive technology outcomes. Am J Obstet Gynecol 211(1):59.e1–8. https://doi.org/10.1016/j.ajog.2014.02.026
Urrutia M, Grinspon RP, Rey RA. 2019. Comparing the role of anti-Müllerian hormone as a marker of FSH action in male and female fertility. Expert Rev Endocrinol Metab 14(3):203–214. https://doi.org/10.1080/17446651.2019.1590197
Vale-Fernandes E, Barreiro M, Leal C, Macedo RZ, Tomé A, Monteiro MP. 2023. Elevated Anti-Müllerian Hormone as a Prognostic Factor for Poor Outcomes of In Vitro Fertilization in Women with Polycystic Ovary Syndrome. Biomedicines 11(12):3150. https://doi.org/10.3390/biomedicines11123150
Visser JA, de Jong FH, Laven JS, Themmen AP. 2006. Anti-Müllerian hormone: a new marker for ovarian function. Reproduction 131(1):1–9. https://doi.org/10.1530/rep.1.00529
Visser JA, Themmen AP. 2014. Role of anti-Müllerian hormone and bone morphogenetic proteins in the regulation of FSH sensitivity. Mol Cell Endocrinol 382(1):460–465. https://doi.org/10.1016/j.mce.2013.08.012

