Available online at: https://doi.org/10.18778/1898-6773.87.4.03
 https://orcid.org/0009-0005-1263-9922
https://orcid.org/0009-0005-1263-9922
Hagerman Fossil Beds National Monument Idaho, Minidoka National Historic Site, Craters of the Moon National Monument and Preserve, National Park Service, Hagerman, ID 83332 USA
 https://orcid.org/0000-0003-3649-3989
https://orcid.org/0000-0003-3649-3989
Dental Microwear Laboratory, Department of Anthropology, College of Arts and Sciences, Georgia State University, Atlanta, GA 30303 USA
ABSTRACT: Introduction: Alongside Australopithecus africanus at Makapansgat South Africa, dated to nearly 3 million years before present, are remnants of Parapapio (Cercopithecinae). The extreme variability of this fossil assemblage has stymied efforts to specify the taxon parameters for Parapapio, which are attributed to at least three species.
Study aims: The first maxillary molar occlusal outlines of the two most complete fossils attributed to Parapapio whitei are compared. The degree of group cohesion in Parapapio whitei is evaluated using three extant cercopithecoid taxa.
Methods and Materials: The fossil crania from Makapangsat Members 3–4, MP 221 and MP 223, both referred to Parapapio whitei, are compared to three extant cercopithecoid taxa including Cercocebus agilis (n=8), Colobus angolensis (n=8) and Papio anubis (n=8). Molar shape is captured using elliptical Fourier analysis of occlusal outlines and molar size dimensions are estimated from measuring software.
Results: MP 223 is larger than MP 221 in occlusal area and the minimum buccolingual length of M1 although the variability between the two Parapapio whitei fossils is commensurate with that observed in Papio anubis. MP 221 and MP 223 are more similar to one another in occlusal outline shape than to any other taxon. However, MP 223 falls consistently closer to Papio anubis whereas MP 221 resembles Papio anubis in some respects and Cercocebus agilis in others.
Conclusion: MP 221 and MP 223 likely belong to a single species with no clear affinity to any of the extant taxa examined. The differences in molar size characterizing Parapapio whitei, a terrestrial forager, is potentially indicative of male bimaturatism or ecological variability which may also characterize Australopithecus africanus at Makapansgat.
KEY WORDS: MP 221, MP 223, Cercocebus agilis, Colobus angolensis, Papio anubis, Australopithecus africanus
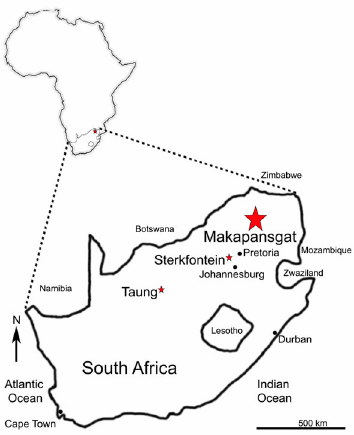
There are at least four cercopithecoid monkeys of the genus Parapapio fossilized in the karstic caves systems of South Africa that yield the remains of early hominins attributed to Australopithecus africanus, ranging from approximately 2.9 to 2.0 Ma before present (BP). These monkey fossils include Parapapio whitei, found primarily at Makapansgat and Sterkfontein, alongside Parapapio jonesi and Parapapio broomi (Fig. 1). A fourth species from South Africa, Parapapio antiquus, has been historically reported at Taung, but may be Procercocebus (Gilbert 2007), or another taxon resembling Cercocebus spp. (Szalay and Delson 1979) (Fig. 1). Parapapio largely becomes extinct in southern Africa around 2 Ma BP with the exception of Parapapio jonesi, which survived the transition to a cool dry environment, and is found in the deposits yielding Paranthropus robustus (Brain 1981; Elton 2007; Williams et al. 2007, cf. Frost et al. 2022).

Figure 1. Location of Makapansgat Cave in South Africa, shown enlarged from a map of Africa
Several approaches have emerged to categorize the fossils attributed to Parapapio. Since the fossilized remains of Parapapio are incomplete, consisting primarily of skull or gnathic fragments encased in breccia, the craniofacial complex and dentition have figured heavily in these investigations (Freedman 1957; 1960; 1976; Maier 1970; Delson 1992; Heaton 2006; Williams et al. 2007; Gilbert 2013). Earlier assessments of Parapapio species attribution relied on the size of the molars, placing Sterkfontein Parapapio whitei as the largest, Pp. jonesi the smallest while Pp. broomi was associated with intermediate values (Freedman and Stenhouse 1972). However, the majority of authors have been equivocal about species attribution within Parapapio (cf. Gear 1926; Jones 1937; Broom and Jensen 1946; Freedman 1957; 1960; 1976; Maier 1970; Freedman and Stenhouse 1972; Eisenhart 1974; Szalay and Delson 1979; Delson 1992; Jablonski 2002; Heaton 2006; Williams et al. 2007; Fourie et al. 2008). Nevertheless, Parapapio whitei appears to present the largest cranial dimensions (Williams et al. 2007) and larger body sizes (Delson et al. 2000) compared to the other southern African species attributed to Parapapio. Given its larger size, Parapapio whitei may have been partly terrestrial, exploiting plants close to ground level or possibly underground storage organs reflected in the mixed C3/C4 isotope signal of this species, at least at Makapansgat (Fourie et al. 2008). Dental microwear texture of Parapapio whitei fossils at Makapansgat, such as MP 223, suggests hard and brittle items as well as adhering grit from underground storage organs were consumed, potentially accounting for the C4 contribution to the diet (Williams 2014). In contrast, the smaller species Pp. jonesi may have had a more folivorous diet at Makapansgat (El-Zaatari et al. 2005).
The nine Parapapio whitei fossils from Makapangat, out of a total of 144 cercopithecoids recovered from the site (Freedman 1976), have been featured in isotopic, dietary, scaling and morphometric studies. Whether any two Parapapio whitei fossils are within the limits of an extant cercopithecoid taxon needs to be addressed prior to examining the attribution of fossils to species. However, as the fossils are fragmentary, comparisons involving morphological size and shape can be challenging.
We chose to examine the molar crown as an indicator of size and shape given the phylogenetic importance of teeth in fossil and living primates. Molar morphology, as well as maximum dimensions of the occlusal surface, are shown to be highly heritable considering the fact that cusp morphology is heavily influenced by genetic factors (Hlusko et al. 2002; 2006; Monson and Hlusko 2014; Paul and Stojanowski 2017). The shape of the occlusal margin of molars is influenced by the maximum and minimum dimensions of the cusps that differ in size per individual and can be effectively captured using elliptical Fourier analysis.
A variety of methods have been developed to describe the shape of the molar crown. These include the use of a generalized Procrustes analysis followed by geometric morphometrics (Gómez-Robles et al. 2007). Generalized Procrustes superimposition compares sets of landmarks to calculate a mean shape rather than a comparison of all landmarks to an arbitrarily designated shape as in elliptical Fourier analysis (Corny and Détroit 2014). Linear distances have also been employed to describe the shape of molars (e.g., Pilloud and Larsen 2011). The scoring of traits using the Arizona State University Dental Anthropology System (Turner et al. 1991; Scott and Irish 2017) is an additional methodological tool invented to describe and compare dental morphology across individuals. These approaches require relatively unworn teeth. Approximating crown form by tracing or landmarking the margin is yet another method to capture the morphology of the molars. In contrast to other approaches, elliptical Fourier analysis of molar occlusal outlines can include individuals with a slight degree of attrition since minor dental wear does not affect the molar margin (Brophy et al. 2014).
Elliptical Fourier analysis creates a numeric description of the shape of an object fitted to a curve by comparing individuals to an idealized sphere (Fig. 2). The deviations between the empirical and standard closed objects are estimated using sine and cosine to calculate the spatial differences between the overlapping ellipses. An outline conforms to the standardized ellipse by increasingly accurate shape modifications. The process continues until the molar outlines start to resemble the standardized ellipse. The sum of these perturbations is quantified into the amplitudes of the harmonics. Each harmonic explains a unique aspect of the shape of an object. Therefore, the more complex the shape, the greater the number of harmonics is needed to describe the object. Any shape can be characterized using elliptical Fourier analysis if the object has a closed contour and is rendered in two dimensions (Lestrel 1974; 1989; Kuhl and Giardina 1982; Iwata and Ukai 2002). A closed contour that is circular or oval, like molars, can be fitted with relatively fewer amplitudes of the harmonics (Ferrario et al. 1999; Corny and Détroit 2014; Williams et al. 2017; 2021).
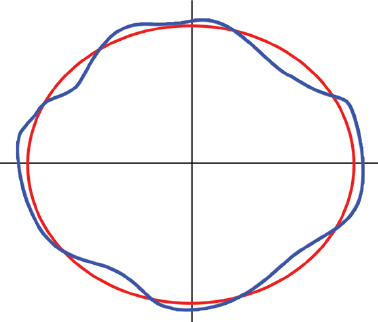
Figure 2. Idealized ellipse (red) compared to a sample (blue) demonstrates how elliptical Fourier functions estimate the difference between standard and empirical closed contours
Another advantage of elliptical Fourier analysis over Procrustes and other morphometric approaches is the ability to compare shapes regardless of size. In a study by Corny and Détroit (2014), Procrustes superimposition and elliptical Fourier analyses were employed to differentiate first and second human molars. Both tools are effective at differentiating individuals into groups (Corny and Détroit 2014; see also Claude 2013). The absence of size or centroid size in the output from elliptical Fourier analysis is critical in this study as the size of the molars of terrestrial monkeys, such as Papio anubis, and some Parapapio fossils are two to three times larger than those of smaller cercopithecine monkeys, such as Cercocebus agilis or African colobine monkeys, such as Colobus angolensis. In this study we utilize elliptical Fourier analysis on the first maxillary molar outlines of MP 221 and MP 223, the two most well preserved, least worn Parapapio whitei fossils from Makapansgat. We ask whether the range of variation between the two fossils attributed to Parapapio whitei are commensurate with that observed in extant cercopithecoid monkeys, including close and more distantly related genera. We expect MP 221 and MP 223 to be distinct from colobine monkeys such as Colobus angolensis, and probably more similar to Papio anubis than to Cercocebus agilis. The inclusion of a species attributed to the Colobinae serves as an outgroup and reflects the fossil cercopithecoid fauna at the site given the presence of the colobine monkey species, Cercopithecoides williamsi, at Makapansgat.
Occlusal outlines and measurements of the first permanent maxillary molar from Parapapio whitei fossils MP 221 and MP 223 were compared to three Old World monkey taxa. These included olive baboons, Papio anubis (n=8) and agile mangabeys, Cercocebus agilis (n=8), which are both cercopithecine monkeys and closely related to Parapapio (Szalay and Delson 1979). We also included Angolan black and white colobus monkeys, Colobus angolensis (n=8) from the Colobinae, which is more distantly related to Parapapio. All three comparative taxa derive from the Democratic Republic of the Congo and were molded at the Royal Museum for Central Africa in Tervuren, Belgium.
The fossil Parapapio whitei remains were examined at the University of the Witwatersrand in Johannesburg, South Africa. Only individuals free of substantial dental attrition, postmortem defects or casting artifacts were included in the study. There are potentially nine relatively well preserved Parapapio whitei fossil crania with gnathic elements from Makapansgat. These include MP 47, MP 76, MP 117, MP 119, MP 221, MP 223, MP 239, M3133 and M3147 (Tab. 1). However, some are reconstructed or damaged areas obscure the taxonomic attribution, while others exhibit extensive dental attrition (Tab. 1).
| Museum# | Museum attribution | Observations |
| MP 47 | not listed | Probable Pp. whitei male given the tall snout, elevated nasal bones and broad palate; teeth heavily worn |
| MP 76 | Pp. whitei | Maier (1970) and Gilbert et al. (2018) refer to Pp. broomi; Freedman (1960, 1976) attributes the cranium to Pp. whitei |
| MP 117 | Pp. whitei | Inferior muzzle with large heavily worn molars |
| MP 119 | Pp. broomi/whitei | Probable Pp. whitei female based on the tall snout and raised nasal bones; extensive dental attrition |
| MP 221 | Pp. whitei | Large well preserved male cranium attributed to Pp. whitei; upper phase 1 breccia west quarry |
| MP 223 | Pp. whitei | Large well preserved male cranium attributed to Pp. whitei; upper phase 1 breccia west quarry |
| MP 239 | Pp. whitei | Likely to be Pp. whitei given the raised nasal bridge; the small molars suggest a female individual, although M1 and M2 exhibit heavy attrition and preservation damage |
| M3147 | not listed | Probable Pp. whitei given the raised nasal bones and tall snout but also shows affinities to fossil Papio sp. from the inflated maxillary fossae |
| M3133 | not listed | Small male, probable Pp. whitei; large and deep palate like other Pp. whitei; molars are damaged and heavily worn |
The individuals ascribed to Parapapio whitei, MP 221 and MP 223, are considered males (Freedman 1976; Williams et al. 2007, Gilbert 2013) and both derive from the upper phase 1 breccia west quarry and were recovered in 1973, corresponding to Members 3–4, but are likely associated with the most fossiliferous zone of the cave consisting of grey breccia and referred to as Member 3. It is also possible that Members 3 and 4 were deposited at overlapping temporal intervals albeit Member 3 may have been more wooded or a shift in the accumulating predators occurred, which likely included hyenas, large felids, porcupines and birds of prey (Reed 1997; Latham et al. 2007; Arbor 2010). Most of the fossils from Makapansgat have no known locality data since they derive from breccia piles left behind during commercial mining operations. In the middle of the 19th century, Makapansgat valley in Limpopo Province of northeast South Africa (Fig. 1) was the site of a Boer raid on a Ndebele tribe whose chief named Mokopane was ambushed near the cave, or gat in Afrikaans (Reed et al. 2022). Bovid fossils from Makapansgat Limeworks were sent to Raymond Dart in 1925 after his famous Taung child was publicized, and some 20 years later, Phillip Tobias and his students collected cercopithecoid fossils from these same breccia piles (Arbor 2010; Reed et al. 2022). When the cercopithecoid fossils from Makapansgat were compared to those recovered from Sterkfontein Member 4 the antiquity and contemporaneity of the two caves was evident to Dart, Tobias and others (Reed et al. 2022). Although dating the Sterkfontein deposits remains mired in uncertainty (cf. Herries et al. 2013; Frost et al. 2022; Granger et al. 2023), Makapangat is probably older given the absence of Papio (Maier 1970; Freedman 1976; Delson 1984; 1988; Jablonski 2002) and other faunal seriations between sites (Vrba 1999; 2000; Reed 1997; Reed et al. 2022).
When the site was formed, Makapansgat was wooded with open areas and presented a mosaic ecology with diverse albeit archaic fauna such as Parapapio whitei (Vrba 1996; 2000; Reed 1997; Elton 2007; Reed et al. 2022). Biochronological estimates using bovid, suid and cercopithecoid fauna date Members 3–4 at Makapansgat to approximately 2.85–2.50 Ma (Herries et al. 2013). A biochronology using cercopithecids gives an estimated date of 2.7–2.6 Ma (Frost et al. 2022) which is identical to a magnetostratigraphy and biochronology study by Warr (2009). Another analysis of paleomagnetism suggests a range of 3.03 to 2.58 Ma (Herries et al. 2013).
Parapapio can be distinguished from Papio by the lack of an ante-orbital drop characterizing the rostrum of extant baboons. The muzzle in Parapapio as viewed in norma lateralis forms a steep slope from glabella to prosthion when preserved (Szalay and Delson 1979; Jablonski 2002). An additional contrast to Papio is the limited degree of sexual dimorphism in Parapapio as well as the smaller overall cranial size (Freedman 1957). The anterior temporal lines are suggested to converge in Parapapio at least in some individuals (Gilbert 2007). Parapapio lacks sagittal cresting and development of the supraorbital torus is minimal; maxillary and mandibular fossae are diminutive or small; and the hypoconulid of M3 is reduced among other distinguishing traits, such as a tall muzzle and raised nasal bones (Gear 1926; Jones 1937; Broom and Jensen 1946; Freedman 1957; 1960; 1976; Maier 1970; Eisenhart 1974; Szalay and Delson 1979; Delson 1992; Jablonski 2002; Heaton 2006; Williams et al. 2007; Gilbert et al. 2018). The teeth are also distinctive in Parapapio as the incisors and molars are smaller than those of Theropithecus darti and larger than those of Cercopithecoides williamsi, at least at Makapansgat (Maier 1970: 89, Table III).
MP 221 comprises a relatively complete cranium from a young adult male, first described by Freedman (1976). Superiorly on the muzzle, the nasal bones are raised. The size of the cranium is relatively large as are the relatively unworn molars, particularly M2 and M3. The canines must have also been impressively large judging from the expansive empty crypts.
MP 223 is probably older than MP 221 given the slightly heavier dental attrition. MP 223 consists of a nearly complete cranium that lacks the superior roof as noted by Freedman (1976). There is a steep facial angle and raised nasal bones typifying Parapapio. The molars are comparatively large. Like MP 221, the rostrum is relatively tall. The canines are also large and somewhat baboon-like.
Dental molds of M1 were created using polyvinylsiloxane, President Jet regular body (Coltène-Whaledent). At the Georgia State University Bioarchaeology Laboratory, the dental molds were placed into putty cradles, catalyzed with activator, prior to being filled with the centrifuged epoxy resin and hardener solution. The dental casts were allowed to cure for at least 24 hours before being pried from the molds.
Toupview® was used to digitize images of the dental casts which were magnified at 30x. Molar occlusal area was approximated using the polygon-tracing tool in which a series of points were placed along the border of molar-to-molar contact to complete an outline (Fig. 3). To standard the images, the first landmark to be digitized was the most lingual aspect of the lingual groove and, continuing clockwise, additional landmarks were placed totaling between 50–60 points. To assess intraobserver error, individuals from each species were traced several times. The resulting differences between tracing attempts were minimal.
The area of the digitized molar outline created in Toupview was calculated using the measurement function. In addition, mesiodistal and minimum buccolingual measurements of the crown were also obtained using the digitized outline. For mesiodistal length of the first maxillary molar, a linear distance between the most mesial and distal extremes was calculated. For the minimum buccolingual distance, the measurement tool was used to draw a line between the most lingual extreme of the intersection of the protocone and hypocone to the midpoint of the buccal margin. A measurement error study was conducted in which two attempts at collecting the measurements were compared using a t-test which yielded nonsignificant differences between trials.
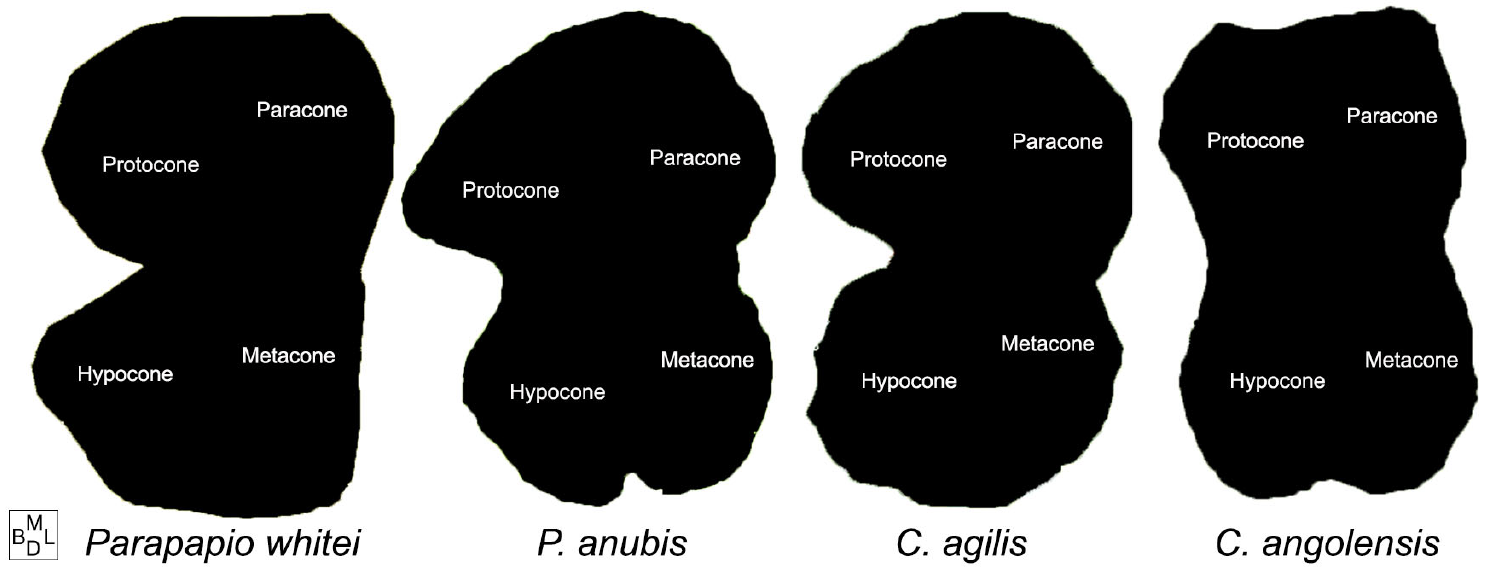
Figure 3. Binarized occlusal outline images of Parapapio whitei (MP 221) compared to Papio anubis, Cercocebus agilis and Colobus angolensis showing the approximate location of the major maxillary cusps on cercopithecoid molars
The digitized outlines from Toupview were imported into photo editing software to binarize the images and to vertically flip any right molars that were traced in place of more poorly preserved left ones. The binarization contrasts the area within the outline, which was rendered black, with the outside of the outline, which was filled with white (Fig. 3).
All of the binarized images (n=26) were subjected to elliptical Fourier analysis within the SHAPE v.2.0 program (Iwata and Ukai 2002). Principal component scores were extracted from the amplitudes of the harmonics to represent molar occlusal outline shape in further analyses. The mean values plus and minus two standard deviations for each principal components vector were visualized to demonstrate the variability of the sample. For the measurements, descriptive statistics for the extant taxa and the values for MP 221 and MP 223 were compared and size differences were further explored in a bivariate plot of mesiodistal and minimum buccolingual lengths. The first PC score, explaining the greatest amount of variation, was contrasted with occlusal area as well as with the second PC axis. In order to encompass a summary of occlusal molar shape variation, a discriminant function analysis was performed on the PC scores identified as significant from the elliptical Fourier descriptors. With a sample size of eight for the comparative taxa, only the first seven PC scores were included so as not to violate the assumptions of the discriminant function analysis. An added benefit of utilizing discriminant function analysis was to classify Parapapio whitei vis-à-vis the extant taxa and to calculate Mahalanobis distances. The first two canonical scores and Mahalanobis distances for MP 221 and MP 223 to all taxa were compared. In all these analyses, the comparative taxa are shown with convex hulls encompassing 100% of the variation in the sample.
Out of 76 PC scores generated in SHAPE, 10 PC scores are identified as significant via elliptical Fourier analysis. There is a sharp decrease in the amount of variance explained after PC5 (5.1%) as demonstrated by the variation around the mean shape of the occlusal outlines (Fig. 4). The amount of variance explained ranges from 34.3% for PC1 to 1.65% for PC10. The first 10 PC scores account for 94%, whereas the first seven explain 87.8% of the total occlusal molar shape variance.
The means and standard deviations for Cercocebus agilis, Colobus angolensis and Papio anubis are compared to the fossil values for mesiodistal and minimum buccolingual lengths and occlusal areas (Tab. 2). Individuals ascribed to Parapapio whitei are larger than Cercocebus agilis and Colobus angolensis for all measurements and are smaller than the Papio anubis mean for mesiodistal length. However, MP 223 rivals the minimum buccolingual mean and exceeds the mean occlusal area for Papio anubis. In contrast, MP 221 is smaller than Papio anubis across the board (Tab. 2).
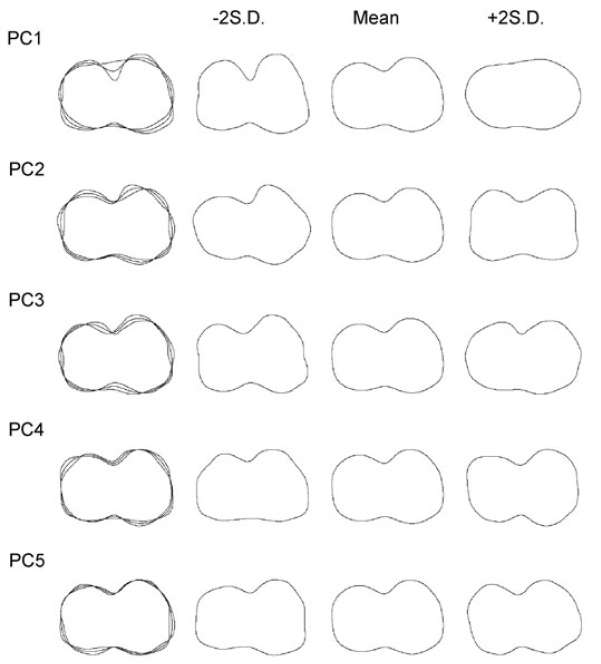
Figure 4. Mean occlusal outlines, plus and minus two standard deviations (S.D.), for the first five PC axes inclusive of 81% of the variance in the sample
| Taxon | Mesiodistal | Minimum Buccolingual | Occlusal area |
| Parapapio whitei (MP 221) | 9.98 | 4.46 | 58.91 |
| Parapapio whitei (MP 223) | 10.01 | 5.77 | 81.71 |
| Cercocebus agilis | 7.018 (0.363) | 3.084 (0.321) | 26.238 (4.208) |
| Colobus angolensis | 6.186 (0.275) | 3.247 (0.166) | 22.571 (1.968) |
| Papio anubis | 11.632 (0.755) | 5.715 (0.484) | 78.444 (11.577) |
The comparison between minimum buccolingual and mesiodistal lengths show that M1 dimensions attributed to Parapapio whitei are much larger than observed in Cercocebus agilis and Colobus angolensis (Fig. 5). Papio anubis exhibits larger dimensions than the other taxa, although MP 223 comes close to the values attributed to Papio anubis. MP 221 and MP 223 are quite similar in mesiodistal length but differ substantially in minimum buccolingual dimensions. However, a similar degree of variation between individuals is observed in Papio anubis (Fig. 5).
When PC1 is contrasted to occlusal area, all of the taxa fall within discrete ranges, including Parapapio whitei represented by MP 221 and MP 223 (Fig. 5). With respect to shape of the maxillary first molar occlusal surface represented by PC1 (34.3%), the difference between the two fossil papionins is relatively small and both fall closest to one Cercocebus agilis individual. This is particularly true of MP 223, which is also similar to a single Papio anubis. Although the difference between the two Parapapio whitei fossils is comparatively large for occlusal area, the same disparity of values can be observed in Papio anubis. Occlusal area can be considered a contrast vector polarizing Colobus angolensis and Papio anubis. Across this axis, MP 221 is only similar to a single Papio anubis while MP 223 is similar to several (Fig. 5).
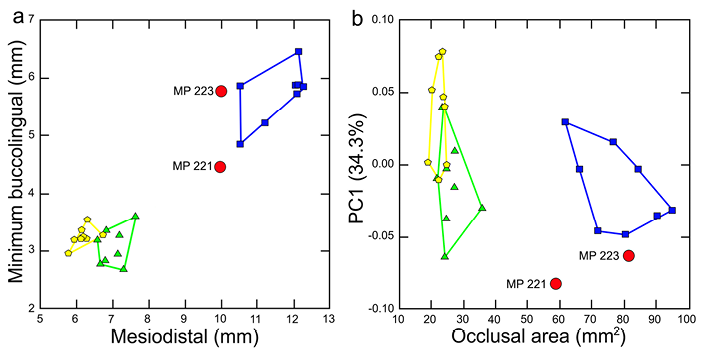
Figure 5. Comparison of (a) minimum buccolingual and mesiodistal M1 lengths and (b) PC1 and occlusal area for Parapapio whitei (red circles); Papio anubis (blue squares); Cercocebus agilis (green triangles) and Colobus angolensis (yellow hexagons)
The first two principal components scores, explaining a total of 52.7% of the variance, completely separate Parapapio whitei from the convex hulls encompassing 100% of the variation in each comparative taxon (Fig. 6). On the first axis, accounting for about a third of the shape variance (34.3%) MP 221, and to a lesser extent MP 223, are separated from Colobus angolensis. Most of the two extant cercopithecines hover close to zero suggesting they are difficult to classify. However, at least one Papio anubis individual falls close to MP 223 suggesting some similarities in shape. In addition, two Cercocebus agilis individuals are close to MP 223 on PC1. However, these same Cercocebus agilis monkeys are polarized from MP 221 on PC2, explaining 18.4% of the variance. Also on the second PC axis, one Colobus angolensis individual approximates the projection of MP 221 whereas MP 223 is undifferentiated from the comparative taxa (Fig. 6).
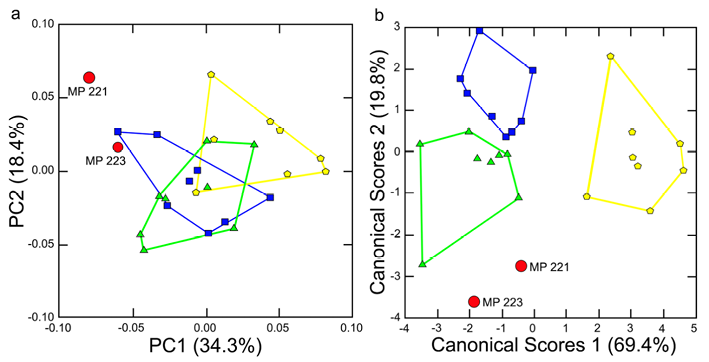
Figure 6. Comparison of (a) the first two PC scores explaining 52.8% of the variance and (b) canonical scores axes 1 and 2 using 7 PC scores for Parapapio whitei (red circles); Papio anubis (blue squares); Cercocebus agilis (green triangles) and Colobus angolensis (yellow hexagons)
The two Parapapio whitei fossils are relatively close to one another and no further away from each other than the extremes of the comparative taxa (Fig. 6). On Canonical Scores 1, with an eigenvalue of 5.7 and explaining 69.4% of the variance, MP 221 and MP 223 are both distinct from Colobus angolensis. However, MP 221 overlaps Cercocebus agilis and Papio anubis. In contrast, MP 223 is similar only to Cercocebus agilis. On Canonical Scores 2 with an eigenvalue of 1.6 and accounting for 19.4% of the shape variation, both MP 221 and MP 223 are decidedly unlike Papio anubis and also differ from Colobus angolensis. In fact, MP 223 is unlike any of the extant taxa on Canonical Scores 2, while MP 221 slightly overlaps one Cercocebus agilis (Fig. 6).
Squared Mahalanobis distances for MP 221 and MP 223 compared to all other taxa demonstrate how well each of these fossil papionins corresponds to their taxon and the relative weights attached to the differences with other taxa. MP 221 and MP 223 exhibit the lowest squared Mahalanobis distances of 5.4 for Parapapio whitei (Table 3). This is much lower than the double digit D2 distances describing the difference between MP 221 and MP 223 and all the comparative taxa. Predictably, the highest D2 distances are between the Parapapio whitei fossils and Colobus angolensis whereas the lowest squared Mahalanobis distance is between Cercocebus agilis and MP 221 of 16.5 (Tab. 3). Relatively low D2 distances also exist between MP 223 and Papio anubis (25.2) and Cercocebus agilis (26). The posterior probabilities for group membership of the Makapansgat fossils with respect to the three comparative taxa are all <0.001. The squared Mahalanobis distances agree with the classification rates in which 96% of individuals are correctly classified. This includes MP 221 and MP 223 which are both classified as Parapapio whitei. However, total jack-knifed classification rates are lower (65%) and one Parapapio whitei is classified as Cercocebus agilis (MP 221) and another as Papio anubis (MP 223).
| C. agilis | C. angolensis | P. anubis | Pp. whitei | |
| MP 221 | 16.5 | 42.9 | 28.1 | 5.4 |
| MP 223 | 26.0 | 33.2 | 25.2 | 5.4 |
We asked whether the morphology captured via occlusal outlines and measurements of the first maxillary molar of MP 221 and MP 223 could be encompassed within a single species of cercopithecoid monkeys. We also asked whether MP 221 and MP 223 are more similar to Papio anubis or to Cercocebus agilis, the two taxa more closely related to early papionins, compared to more distantly related colobine monkeys represented by Colobus angolensis.
MP 221 and MP 223 are similar mesiodistally but differ substantially in minimum buccolingual dimensions and occlusal area. The difference in molar size between MP 221 and MP 223 has been commented on previously by Freedman (1976) who first described these two fossils. In fact, the difference was so pronounced with respect to other cercopithecoid comparisons that Freedman (1976) equivocated as to whether MP 221 was actually Parapapio whitei, proposing the possibility that this fossil might also be Pp. broomi. However, a similar molar size difference between individuals is seen in Papio anubis (Fig. 5) suggesting MP 221 and MP 223 could be members of the same species. The same could be said for shape (Fig. 6). None of the resulting size and shape comparisons of MP 221 and MP 223 with the three cercopithecoid species refute the association of these two males as belonging to a single taxon.
The taxonomy of the genus Parapapio has come under scrutiny. For example, Parapapio antiquus could be a subspecies of Pp. broomi (Heaton 2006) or a different genus, Procercocebus, purportedly ancestral to the Cercocebus clade (Gilbert 2007). Shape resemblances between Parapapio and Papio (Freedman 1957) and Parapapio and Cercocebus spp. (Szalay and Delson 1979) have been noted previously. With respect to the results obtained here, the question of whether MP 221 and MP 223 are likely to be ancestral to Papio typified by Papio anubis or a Cercocebus clade represented by Cercocebus agilis is uncertain. MP 223 is clearly more aligned with Papio anubis in some ways, but does not fall within the convex hull for this species in size and shape indices (Fig. 5 and 6) and squared Mahalanobis distances present significant differences with extant taxa. The case for MP 221 is more complex in that there is some similarity in occlusal outline shape of M1 with Cercocebus agilis and perhaps, to a lesser degree, with Papio anubis. Parapapio whitei and other taxonomic labels given to the fossil primate assemblage of Makapansgat may represent a primate taxon in the process of speciation. The variation in early hominins from this site is also pronounced (Arbor 2010). Much of this variation aligns with Australopithecus africanus while other aspects such as mandibular corpus size in MLD 40 and molar size and crown complexity in MLD 2 resemble Paranthropus robustus, and still other morphological features that are unique to the Makapansgat hominin assemblage (Aguirre 1970; Tobias 1980; Arbor 2010). Given the large mandibular corpus dimensions in both MLD 2 and MLD 40, they are likely to be males (although the former is a subadult). The males attributed to Parapapio whitei, MP 221 and MP 223, are similarly variable in M1 dimensions (Tab. 2; Fig. 5). Cranial vault size differences between Parapapio whitei M3133 and MP 223 further argues for bimaturism among males (Tab. 1).
The relatively larger size of Parapapio whitei (Delson et al. 2000) could imply a greater degree of terrestrial foraging than other taxa of the genus. At Sterkfontein Member 4, Parapapio whitei appears to have consumed more grasses and leaves than fruit compared Pp. broomi and Pp. jonesi (Benefit 1999). At Makapansgat, the diet of Parapapio whitei specimen MP 223 indicates hard and brittle foods or adhering grit were consumed, potentially from underground storage organs (Williams 2014) which could account for the partial C4 signal in the diet (Fourie et al. 2008). Indeed, the ecological processes that drove increasingly greater terrestriality in Parapapio could have also operated on Australopithecus africanus.
The two best preserved adult Parapapio whitei males, MP 221 and MP 223, of different sizes fall in between semi-terrestrial Papio anubis and tropical forest floor hard-object specialists typified by Cercocebus agilis. The differences between these two habitat preferences, tropical forest and woodlands, also seem to characterize reconstructions of the mosaic landscape of Pliocene Makapansgat, comprising wooded habitats interspersed by open areas with sources of water such as rivers in proximity (Reed et al. 2022). In this southern African habitat during the late Pliocene, Parapapio whitei and Australopithecus africanus likely competed for the same C3/C4 resources reflecting the mixed carbon isotopic signature characterizing both species (Codron et al. 2005). The two species probably fled from the same predators and contested vital resources such as sleeping sites (Benefit 1999). Although other primates such as Theropithecus darti, existed at Makapansgat, Pp. whitei is the largest species of the plentiful assortment of fossil cranial fragments attributed to Parapapio (Freedman 1976; Delson et al. 2000). Its larger size placed it in more direct competition with early hominins such as Australopithecus africanus.
Parapapio whitei exhibits a relatively long M1 that is as variable in minimum buccolingual dimensions and occlusal area as observed in Papio anubis. Yet this difference is between two males with relatively large canines, suggesting bimaturism or some other mechanism may account for the evolution of such intrasexual variability. The first maxillary molar outlines of the only two well-preserved individuals, that happen to be males, attributed to Parapapio whitei from Makapansgat present a range of variation that resembles what is observed in Papio anubis. With respect to both size and shape, MP 223 is more similar to Papio anubis while MP 221 resembles both P. anubis and Cercocebus agilis. However, squared Mahalanobis distances group the two fossils to Parapapio whitei and significantly differentiate them from all extant taxa suggesting these two males derive from the same taxon.
Parapapio whitei was the largest taxon of the genus in southern Africa and thus probably among the most terrestrial of the primate fauna living in the same habitats as Australopithecus africanus. Parapapio whitei and Australopithecus africanus probably competed for the same foods and other resources (Benefit 1999; Elton 2007). The difference in size between the two Parapapio whitei males presents the possibility that ecological variability, intrasexual competition or bimaturism could have evolved. The same processes could have also led to large intrasexual size distinctions in other semi-terrestrial primates, such as Australopithecus africanus.
Acknowledgments
We thank Mike Raath and Philip Tobias (posthumously) at the University of the Witwatersrand and Emmanuel Gilissen at the Royal Museum for Central Africa for permission to examine the material in their care. We also thank Victoria Seabolt for rerunning the analysis in SHAPE and William Anderson for assistance with creating the epoxy resin dental casts. Funding was received from Fulbright-Belgium and the Commission for Educational Exchange between the US, Belgium, and Luxembourg as well as a Professional Enhancement Grant and a Student Technology Fee Award from Georgia State University.
Conflict of interests
The authors have no conflicts of interest to declare. An employee may use, or permit the use of, his/her title in connection with an article published in a scientific or professional journal, provided that the title or position is accompanied by a reasonably prominent disclaimer stating that the views expressed in the article are the employee’s and do not represent the Government.
Authors’ contribution
ACK created the outlines and measurements of the dental casts and contributed to the writing and editing. FLW conceptualized the work; created the dental molds and casts and led the writing of the paper.
Aguirre E. 1970. Identificacion de “Paranthropus” en Makapansgat. In: Anonymous XI Congreso Nacional de Arqueología. Zaragoza: Octavio y Féliz. 98–124.
Arbor T. 2010. The Makapansgat hominins: a descriptive and comparative morphological study. Ph.D. dissertation, Washington University, St. Louis.
Benefit BR. 1999. Biogeography, dietary specialization and the diversification of African Plio-Pleistocene monkeys. In: TG Bromage and F. Schrenk, editors. African Biogeography, Climate Change, and Human Evolution. Oxford: Oxford University Press. 172–188.
Brain CK. 1981. The Hunters or the Hunted? An Introduction to African Cave Taphonomy. Chicago: University of Chicago Press.
Broom R, Jensen JS. 1946. A new fossil baboon from the caves at Potgietersrust. Ann Transvaal Mus 20:337–340.
Brophy JK, de Ruiter DJ, Athrey AS, de Witt TJ. 2014. Quantitative morphological analysis of bovid teeth and implications for paleoenvironmental reconstruction of Plovers Lake, Gauteng Province, South Africa. J Archaeol Sci 41:376–388. http://doi.org/10.1016/j.jas.2013.08.005
Bryan BC, Williams FL. 2021. Comparing maxillary first molar crown shape using elliptical Fourier analysis in the Late Neolithic cave burials of Belgium. Anthropol Rev 84(1):1–15. https://doi.org/10.2478/anre-2021-0001
Claude M. 2013. Log-shape ratios, Procrustes superimposition, Elliptic Fourier Analysis: three worked examples in R. Hystrix 24(1):94–102. https://doi.org/10.4404/hystrix-24.1-6316
Codron D, Luyt J, Lee-Thorp JA, Sponheimer M, De Ruiter D, Codron J. 2005. Utilization of savanna-based resources by Plio-Pleistocene baboons. S Afri J Sci 101(5–6):245–248. https://doi.org/10.5167/uzh-25352
Corny J, Détroit F. 2014. Technical Note: Anatomic identification of isolated modern human molars: testing Procrustes aligned outlines as a standardization procedure for elliptic Fourier analysis. Am J Phys Anthropol 153(2):314–322. https://doi.org/10.1002/ajpa.22428
Delson E. 1984. Cercopithecid biochronology of the African Plio-Pleistocene: correlations among eastern and southern hominid-bearing locaties. Cour Forsch-Inst Seneckenberg 69:199–218.
Delson E. 1988. Chronology of South African Australopith site units. In: FE Grine, editor. Evolutionary History of the Robust Australopithecines. New York: Aldine. 317–324.
Delson E. 1992. Evolution of Old World monkeys. In: JS Jones, RD Martin, D Pilbeam and S Bunney, editors. The Cambridge Encyclopedia of Human Evolution. Cambridge: Cambridge University Press. 217–222.
Delson E, Terranova CJ, Jungers WL, Sargis EJ, Jablonski NG, Dechow PC. 2000. Body Mass in Cercopithecidae (Primates, Mammalia): Estimation and Scaling in Extinct and Extant Taxa. New York: Anthropological Papers of the American Museum of Natural History (Vol. 83).
Elton E. 2007. Environmental correlates of the cercopithecoid radiations. Folia Primatol 78:244–364. http://doi.org/10.1159/000105149
Eisenhart WL. 1974. The fossil cercopithecoids of Makapansgat and Sterkfontein. Unpublished B.A. Thesis, Harvard University.
El-Zaatari S, Grine FE, Teaford MF, Smith HF. 2005. Molar microwear and dietary reconstructions of fossil cercopithecoidea from the Plio-Pleistocene deposits of South Africa. J Hum Evol 49(2):180–205. https://doi.org/10.1016/j.jhevol.2005.03.005
Ferrario VF, Sforza C, Tartaglia GM, Colombo A, Serrao G. 1999. Size and shape of the human first permanent molar: a Fourier analysis of the occlusal and equatorial outlines. Am J Phys Anthropol 108:281–294. https://doi.org/10.1002/(SICI)1096-8644(199903)108:3<281::AID-AJPA4>3.0.CO;2
Fourie NH, Lee-Thorp JA, Ackermann RR. 2008. Biogeochemical and craniometric investigation of dietary ecology, niche separation, and taxonomy of Plio-Pleistocene cercopithecoids from the Makapansgat limeworks. Am J Phys Anthropol 135:121–135. http://doi.org/10.1002/ajpa.20713
Freedman L. 1957. The fossil cercopithecoidea of South Africa. Ann Transvaal Mus 23:122–262.
Freedman L. 1960. Some new fossil cercopithecoid specimens from Makapansgat, South Africa. Palaeont Afr 7:7–45.
Freedman L. 1976. South African fossil Ceropithecoidea: a reassessment including a description of new material from Makapansgat, Sterkfontein and Taung. J Hum Evol 5:297–315.
Freedman L, Stenhouse NS. 1972. The Parapapio specimens of Sterkfontein, Transvaal, South Africa. Palaeont Afr 14:93–111.
Frost SR, White FJ, Reda HG, Gilbert CC. 2022. Biochronology of South African hominin-bearing sites: a reassessment using cercopithecid primates. Proc Natl Acad Sci USA 119(45):e2210627119. http://doi.org/10.1073/pnas.2210627119
Gear JHS. 1926. A preliminary account of the baboon remains from Taungs. S Afr J Sci 23:731–747.
Gilbert CC. 2007. Craniomandibular morphology supporting the diphyletic origin of mangabeys and new genus of the Cercocebus/Mandrillus clade, Procercocebus. J Hum Evol 53(1):69–102. https://doi.org/10.1016/j.jhevol.2007.03.004
Gilbert CC. 2013. Cladistic analysis of extant and fossil African papionins using craniodental data. J Hum Evol 64(5):399–433. https://doi.org/10.1016/j.jhevol.2013.01.013
Gilbert CC, Frost SR, Pugh KD, Anderson M, Delson E. 2018. Evolution of the modern baboon (Papio hamadryas): a reassessment of the African Plio-Pleistocene record. J Hum Evol 122:38–69. https://doi.org/10.1016/j.jhevol.2018.04.012
Gómez-Robles A, Marinón-Torres M, Bermúdez de Castro JM. 2007. A geometric morphometric analysis of hominin upper first molar shape. J Hum Evol 53:272–285. http://doi.org/10.1016/j.jhevol.2007.02.002
Granger DE, Stratford D, Bruxelles L, Heaton JL, Pickering TR, Kuman K, Clarke RJ. 2023. Monkey fossils do not negate cosmogenic dating at Sterkfontein. Proc Natl Acad Sci USA. 120(13):e2300314120. http://doi.org/10.1073/pnas.2300314120
Heaton JL. 2006. Taxonomy of the Sterkfontein fossil Cercopithecinae: the Papionini of Members 2 and 4 (Guateng, South Africa). Ph.D. dissertation, Indiana University, Bloomington.
Herries AIR, Pickering R, Adams JW, Curnoe D, Warr G, Latham AG, et al. 2013. A multi-disciplinary perspective on the age of Australopithecus in southern Africa. In: K Reed, J Fleagle and R Leakey, editors. The Paleobiology of Australopithecus. Vertebrate Paleobiology and Paleoanthropology. Dordrecht: Springer. 21–40. http://doi.org/10.1007/978-94-007-5919-0_3
Hlusko LJ, Do N, Mahaney MC. 2006. Genetic correlations between mandibular molar cusp areas in baboons. Yearb Phys Anthropol 49:2–48. https://doi.org/10.1002/ajpa.20528
Hlusko LJ, Weiss KM, Mahaney MC. 2002. Statistical genetic comparison of two techniques for assessing molar crown size in pedigreed baboons. Am J Phys Anthropol 117(2):182–189. https://doi.org/10.1002/ajpa.10022
Iwata H, Ukai Y. 2002. SHAPE: A computer program package for quantitative evaluation of biological shapes based on elliptic Fourier descriptors. J Heredity 93:384–385. http://doi.org/10.1093/jhered/93.5.384
Jablonski NG. 2002. Fossil Old World monkeys: the Late Neogene radiation. In: WC Harwig, editor. The Primate Fossil Record. Cambridge: Cambridge University Press. 225–300.
Jones TR. 1937. A new fossil primate from Sterkfontein, Krugersdorp, Transvaal. S Afr J Sci 33:709–728.
Kuhl FP, Giardina CR. 1982. Elliptic Fourier features of a closed contour. Comput Gr Image Process 18:236–258.
Latham AG, McKee JK, Tobias PV. 2007. Bone breccias, bone dumps, and sedimentary sequences of the western Limeworks, Makapansgat, South Africa. J Hum Evol 52(4):388–400. http://doi.org/10.1016/j.jhevol.2006.10.005
Lestrel PE. 1974. Some problems in the assessment of morphological shape differences. Yearb Phys Anthropol 18:140–162.
Lestrel PE. 1989. Method for analyzing complex two-dimensional forms: elliptical Fourier functions. Am J Hum Biol 1:149–164. http://doi.org/10.1002/ajhb.1310010204
Maier W. 1970. New fossil Cercopithecoidea from the lower Pleistocene cave deposits of the Makapansgat limeworks, South Africa. Palaeont Afr 13:69–107.
Monson TA, Hlusko LJ. 2014. Identification of a derived dental trait in the Papionini relative to other Old World monkeys. Am J Phys Anthropol 155(3):422–429. https://doi.org/10.1002/ajpa.22586
Paul KS, Stojanowski CM. 2017. Comparative performance of deciduous and permanent dental morphology in detecting biological relatives. Am J Phys Anthropol 164:97–116. http://doi.org/10.1002/ajpa.23260
Pilloud MA, Larsen CS. 2011. “Official” and “practical” kin: inferring social and community structure from dental phenotype at Neolithic Çatalhöyük, Turkey. Am J Phys Anthropol 145:519–530. http://doi.org/10.1002/ajpa.21520
Reed KE. 1997. Early hominid evolution and ecological change through the African Plio-Pleistocene. J Hum Evol 32(2–3):289–322. http://doi.org/10.1006/jhev.1996.0106
Reed KE, Kuykendall KL, Herries AIR, Hopley PJ, Sponheimer M, Werdelin L. 2022. Geology, fauna, and paleoenvironmental reconstructions of the Makapansgat Limeworks Australopithecus africanus-bearing paleo-cave. In: SC Reynolds and R Bobe, editors. African Paleoecology and Human Evolution. Cambridge: Cambridge University Press. 66–81.
Scott GR, Irish JD. 2017. Human Tooth Crown and Root Morphology. Cambridge: Cambridge University Press.
Szalay FS, Delson E. 1979. Evolutionary History of the Primates. New York: Academic Press.
Tobias PV. 1980. “Australopithecus afarensis” and A. africanus: Critique and alternative hypothesis. Palaeontol Afr 23:1–17.
Turner II, CG, Nichol C, Scott GR. 1991. Scoring procedures for key morphological traits of the permanent dentition: the Arizona State University Dental Anthropology System. In: MA Kelley and CS Larsen, editors. Advances in Dental Anthropology. New York: Wiley-Liss. 13–31.
Vrba ES. 1996. Habitat theory in relation to the evolution in African Neogene biota and hominids. In: TG Bromage and F Schrenk, editors. African Biogeography, Climate Change, & Human Evolution. New York: Oxford University Press. 19–46.
Vrba ES. 2000. Major features of Neogene mammalian evolution in Africa. In: TC Partridge and RR Maud, editors. The Cenozoic of Southern Africa. New York: Oxford University Press. 277–304.
Warr GL. 2009. Chronology of the western Limeworks australopithecine site, Makapansgat, South Africa: magnetostratigraphy, biochronology and implications for hominin evolution. Ph.D. Thesis, University of Liverpool.
Williams FL. 2014. Dietary reconstruction of Pliocene Parapapio whitei from Makapansgat, South Africa, using dental microwear texture analysis. Folia Primatol 85:21–37. http://doi.org/10.1159/000356029
Williams FL, Ackermann RR, Leigh SR. 2007. Inferring Plio-Pleistocene southern African biochronology from facial affinities in Parapapio and other fossil papionins. Am J Phys Anthropol 132:163–174. https://doi.org/10.1002/ajpa.20504
Williams FL, Lane KM, Anderson WG. 2017. Comparison of maxillary first molar occlusal outlines of Neandertals from the Meuse River Basin of Belgium using elliptical Fourier analysis. Anthropol Rev 80:273–286. https://doi.org/10.1515/anre-2017-0018

