.jpg)
Available online at: https://doi.org/10.18778/1898-6773.87.4.01
 https://orcid.org/0009-0006-8890-4179
https://orcid.org/0009-0006-8890-4179
Department of Anthropology, West Bengal State University, Barasat, West Bengal 700126, India
 https://orcid.org/0000-0001-6129-8486
https://orcid.org/0000-0001-6129-8486
Department of Anthropology, Mrinalini Datta Mahavidyapith, Birati, Kolkata, West Bengal, India
ABSTRACT: After attaining menarche adolescents, due to shyness and fear, often refuse to seek medical treatment. Simultaneously they began to face menstrual disorders. The present review aimed to estimate the overall menstrual disorders and associated factors among adolescent girls in rural and tribal areas in India as well as to summarize the most recent research findings on the pooled prevalence of menstrual disorders. The study design was developed applying the PRISMA checklist-2020. The whole protocol was registered on PROSPERO (Registration ID: CRD42024385046). Articles (English language) related to menstrual irregularities among 10 to 19-year-old adolescent girls in India were collected based on inclusion and exclusion criteria from 2000 to 2023 followed by selected keywords. The quality assessment of the present study was evaluated using the CASP (Critical Appraisal Skills Programme) checklist. Meta-analysis was conducted by using MedCalc software version 22.0. Publication bias was checked using Egger’s test. A total of 61 studies (47 from rural and 14 from tribal areas) in India have been evaluated. The random effect model showed an overall prevalence of dysmenorrhea, irregular menstruation, PMS, oligomenorrhea, polymenorrhea and menorrhagia in both areas was 54.96% (95% CI: 47.93 to 61.85), 26.21% (95% CI: 20.73 to 32.09), 47.49% (95% CI: 31.44 to 63.81), 13.88% (95% CI: 8.98 to 19.65),7.85% (95% CI: 2.30 to 16.31), 16.83% (95% CI: 10.04 to 24.96) respectively. Among these, dysmenorrhea, irregular menstruation, and PMS were found to be the most predominant in both areas. Lack of physical activities, dietary habits, BMI, socioeconomic factors, and socio-cultural taboos were found to have a strong association with menstrual irregularities. Prior and after attaining menarche, proper guidance on every aspect of menstruation should be urgently arranged in schools and at home to get rid of fear and anxiety, so that adolescent girls can cope with menstrual-related issues. Health camps should be organized in both areas to allow an easy access.
KEY WORDS: menstrual disorders, associated factors, adolescent girls, rural, tribal, India, systematic review, meta-analysis
Menstruation is a health issue that has three dimensions: a physiological, psychological, and social process that needs to be addressed before menarche and after menopause (WHO 2022). Adolescent girls are often not aware of menarche hence the first period can be accompanied by fear, and anxiety along with stigma, taboos, and myths (UNICEF, 2018). Because of these myths and misconceptions about menstruation, girls often develop negative attitudes towards this pubertal development process (Walia et al. 2015). Being a secret and personal matter, mothers used to merely talked about this aspect of life hence friends were the sources of information about menarche (Dhingra et al. 2009). Dysmenorrhea or abdominal cramps, pre-menstrual syndromes (PMS), and irregular menstruation were the most common disorders among adolescent girls in rural areas of West Bengal (Sanyal and Ray 2008; Ray et al. 2010). More than 70% of tribal adolescent girls were experiencing dysmenorrhea (Nagar and Aimol 2010; Shanmugananth et al. 2023). By neglecting this issue, menstrual disorders have been increasing, especially among late adolescent girls (Singh and Kasturwar 2017; Sharma et al. 2019). Interestingly, a recent systematic review on menstrual hygiene practices and menstrual morbidities among adolescent girls in India showed that the most prevalent disorders were dysmenorrhea, PMS, oligomenorrhea, menorrhagia and polymenorrhea (Majeed et al. 2022). It has been reported that about 64% of adolescent girls experience at least one type of problem-related to menstruation (Pearlstein and Steiner 2008).
Nowadays there are lot of changes in the daily lifestyle of adolescents. Dietary habits and physical activity are the two major concerns that were significantly associated with disorders during menstruation (Negi et al. 2018). The daily routine was interrupted due to menstrual irregularities, resulting in prolonged bed rest, sleep disturbances along school absenteeism (Sharma et al. 2008). Nutritional status and socio-economic condition were associated with menstrual irregularities (Verma et al. 2021). The age of the respondents was also associated with menstrual-related problems (Sanyal and Ray, 2008; Nagar and Aimol 2010). Menstrual disorders also had a significant association with family history (Kumbhar et al. 2011).
Systematic reviews generally have a detailed search strategy by synthesizing all relevant articles on a particular topic to reduce publication bias. That feature makes a systematic review more reliable and different from a narrative review (Uman 2011). Systematic review is usually done to reduce random errors and bias. Sometimes systematic review comes with meta-analysis, which involves a statistical technique to create all quantitative data retrieved from all the studies into single or combined results to give a clear idea about the particular topic (Petticrew and Roberts 2008).
Although there many reviews have been published on Menstrual Hygiene Management, among urban, rural as well as tribal adolescent girls in India, no reviews, to our knowledge, have focused on menstrual disorders among rural and tribal adolescent girls in India till now. Hence this review is very important to identify the menstrual problems among rural and tribal India as well as associated factors during menstruation that can be linked with Sustainable Development Goals (SDGs), specifically, SDG 3 states “Good Health and Well Being”.
The whole protocol of the present review was registered on PROSPERO (Registration ID: CRD42024385046).
Data extraction and inclusion were done based on previously framed inclusion and exclusion criteria (Tab. 1).
| Inclusion criteria | Exclusion criteria |
| 1. Articles were limited to India only. | 1. Beyond India. |
| 2. Studies related to 2000–2023. | 2. Before 2000 and after 2023. |
| 3. Targeted population aged between 10–19 years among rural and tribal areas only. | 3. Adolescents aged less than 10 years and over 19 years or adult population or belonging to the urban areas. |
| 4. Clearly defined community-based cross-sectional studies related to menstrual disorders. | 4. Review papers, short articles, letters to editors, disorders vaguely mentioned articles. |
| 5. Comparative study. | 5. Case-control and intervention-based study. |
The study design was developed in line with the “preferred reporting items for systematic reviews and meta-analysis” (PRISMA) checklist (Page et al. 2021). Studies published in English language from the year 2000 to 2023 focusing on the prevalence of menstrual disorders among 10 to 19-year-old adolescent girls from rural and tribal areas in India were included. Data were collected from the following databases: PubMed Central, NCBI, Research Gate, Academia and Google Scholar, Science Direct, BMC, and PLOS ONE. Some keywords from MeSH have been used to make this review more reliable and combined through the Boolean operator (“AND”, “OR”) i.e, “menstrual disorders” OR “menstrual irregularities” OR “menstrual health status” OR “menstrual patterns” OR “menstrual disturbances”, “dysmenorrhoea” OR “abdominal cramps” OR “pain in the abdomen”, “menorrhagia” OR “heavy menstrual bleeding”, “irregular menstruation” OR “irregular menses” OR “irregular cycle”, “oligomenorrhea” AND “polymenorrhea”, AND “amenorrhea”, AND “hypomenorrhea”, AND “pre-menstrual syndromes”, AND “adolescent girls”, AND “rural”, AND/OR “tribal”, “India”, “cross-sectional” AND “Association” OR “predictors”. PRISMA checklist for systematic reviews has been followed throughout this review, presented in a supplementary file.
An initial search of a total of 350 (abstract and full text) articles was carried out from databases such as PubMed Central, NCBI, Research Gate, Academia, Google Scholar, Science Direct, BMC, and PLOS ONE. Cross references were also considered in searching for relevant articles. Based on inclusion and exclusion criteria, a total of 289 articles have been extracted. After the final screening, a total of 61 articles (47 from rural, 14 from tribal areas, and 1 paper belonging to rural and tribal areas) among Indian adolescent girls have been included. A flow diagram of selecting articles is depicted in Figure 1. To construct keyword co-occurrences a networking map was created by using an online software tool “VOSviewer” version 1.6.20 (Fig. 2). The greatest number of co-occurrences of keywords were adolescents, menstruation, menstrual disturbances, and India (Fig. 2).
.jpg)
Figure 1. PRISMA flow diagram of the present systematic review
.png)

Figure 2. Keyword co-occurrences of included studies
The quality assessment was evaluated by the CASP (Critical Appraisal Skills Programme) systematic review checklist as shown in the supplementary file.
All included studies were based on secondary data, hence ethical approval is not needed.
Meta-analysis was done by using MedCalc software version 22.0.
Heterogeneity within studies included in the meta-analysis was represented by a forest plot and publication bias in the present study was represented through a funnel plot or scatter plot. The test of heterogeneity was done by Cochrane’s Q statistic-based I2 statistic. Scores of heterogeneities are measured by three types, i.e., 25% (low), 50% (moderate), and 75% (high heterogeneity). The study is considered homogenous when I2<50% and thus a fixed effect model is considered. P value<0.1 or I2 value> 50% indicates heterogeneity and thus a random effect model (DerSimonian and Laird, 1986) should be used to reduce bias. Heterogeneity scores in this study were above 50%, hence we used the random effect model. Each study was represented through a black square and a horizontal line (95%CI). The aggregate effect size was displayed by the diamond at the bottom.
Publication bias was assessed using Egger’s test. An intercept with p<0.05 indicates publication bias.
The date of the first submission of this review protocol in PROSPERO was February 11, 2024, and the registration date in PROSPERO was February 22, 2024. Following that, starting from preliminary searches, data analysis, and manuscript writing took almost 3 months, i.e., March to May.
Table 2 and Table 3 show various menstrual disorders among adolescent girls in rural and tribal areas. Both tables show sample size, age group, study design, and prevalence of various menstrual disorders found in rural and tribal study areas. A total of 61 studies (47 from rural, 14 from tribal areas, and 1 paper belonging to rural and tribal areas) among adolescent girls aged between 10 to 19 years in India have been evaluated and included in the analysis. In rural areas, sample size varied between 50 (Dharani and Sood, 2018) to 958 (Kohli and Kapoor 2021), whereas in tribal areas it varied between 100 (Nagar and Aimol 2010) to 507 (Shanmuganath et al. 2023).
Figure 3 shows the forest plot of the prevalence of dysmenorrhea. Each horizontal line with a square represents each included study. A total of 48 studies from rural and tribal adolescent girls in India were included in the stipulated time. The diamond at the bottom represents the overall results with a 95% confidence interval. The pooled prevalence of dysmenorrhea in rural areas was found the highest (89.33% with 95% CI: 80.05 to 95.27) by Das et al. (2019), while the lowest prevalence (12.80% with 95% CI: 10.74 to 15.08) was found by Kohli and Kapoor (2021). In tribal areas the highest prevalence of dysmenorrhea (78.30% with 95% CI: 74.45 to 81.81) was found in the study by Shanmugananth et al. (2023) and the lowest (15.80% with 95% CI: 11.71 to 20.63) was found by Kakeri et al. (2018). The overall prevalence of dysmenorrhea was 54.94% (95% CI: 47.93 to 61.85). Heterogeneity scores in this study were above 50% (Q-3374.99, DF-47, p<0.0001, I2-98.61%), hence random effect model was considered. Figure 4 shows the funnel plot of effect size against sample size. Evidence of publication bias has been found through Egger’s test (p=0.0078).
Figure 5 shows the forest plot of the prevalence of irregular menstruation. A total of 42 studies from rural and tribal adolescent girls in India were included. The diamond at the bottom represents the overall results with a 95% confidence interval. Analysis showed the highest prevalence of irregular menstruation in rural areas was found at 95.38% (95% CI: 92.49 to 97.39) by Ray et al. (2010), while the lowest prevalence was found at 5.60% (95% CI: 3.35 to 8.69) in the study by Kanotra et al. (2013). In tribal areas, the highest prevalence of irregular menstruation (32.30% with 95% CI: 28.24 to 36.56) was found in the study reported by Shanmugananth et al. (2023), and the lowest prevalence (12.60% with 95% CI: 7.74 to 18.99) was found by Kale et al. (2023). The random effect model showed that the prevalence of irregular menstruation was 26.21% (95% CI: 20.73 to 32.09). Substantial heterogeneity was found (Q-2232.65, DF-42, p<0.0001, I2-98.12%). Figure 6 shows a funnel plot of the included studies and indicates no publication bias through Egger’s test (p=0.931).
| Study area | Studied sample size | Studied age group (in years) | Study design | Menstrual disorders | References | |||||||
| Dysmenorrhea | Irregular menstruation | PMS | Oligomenorrhea | Polymenorrhea | Amenorrhea | Menorrhagia | hypomenorrhea | |||||
| 1. Garhwal | 470 | 13–19 | Cross-sectional | 62.76% | 28.72% | 40.42% | - | - | - | 9.50% | - | Negi et al. 2018 |
| 2. Amravati, Maharashtra | 435 | 12–16 | Prospective observational | 62.30% | 21.80% | 17.90% | - | - | - | - | - | Wasnik et al. 2015 |
| 3. Tamil Nadu | 500 | 14–19 | Cross-sectional | 65.00% | - | 62.20% | 16.00% | - | - | 11.00% | - | Priya et al. 2016 |
| 4. Nagpur | 146 | 12–16 | Cross sectional | 41.78% | - | 34.90% | - | - | - | - | - | Thakre et al. 2012 |
| 5. Pondicherry | 190 | 11–18 | Cross-sectional | 52.02% | 24.00% | - | - | - | - | - | - | Karthiga et al. 2011 |
| 6. Lucknow | 254 | 10–19 | Cross-sectional | 72.60% | 18.10% | - | - | - | - | - | - | Sachan et al. 2012 |
| 7. Lucknow | 176 | 1019 | Cross-sectional | 72.70% | 18.10% | - | - | - | - | - | - | Sinha et al. 2016 |
| 8. Bhopal | 400 | 12–19 | Cross-sectional | 33.75% | 18.50% | - | 8.00% | 24.75% | - | - | - | Patel et al. 2023 |
| 9. Tamil Nadu | 350 | 10–19 | Cross-sectional | 72.60% | 31.70% | - | - | - | - | 45.70% | - | Ravi et al. 2016 |
| 10. Bijapur | 440 | 11–16 | Cross-sectional | 28.00% | 7.50% | - | - | 0.45% | 5.90% | - | Patil and Angadi 2013 | |
| 11. Wardha | 171 | 10–19 | Cross-sectional | 45.61% | 31.57% | 65.50% | - | - | - | - | - | Dambhare et al. 2012 |
| 12. Varanasi | 240 | 12–18 | Cross-sectional | 82.50% | 37.00% | - | - | - | - | - | - | Nagar et al. 2022* |
| 13. Dharwad, Karnataka | 422 | 13–19 | Cross-sectional | 36.00% | - | - | - | - | - | - | - | Rajaretnam et al. 2010 |
| 14. Haryana | 300 | 17–19 | Cross-sectional | 78.30%** | 33.96% | - | 16.67% | 6.67% | 2.33% | 9.00% | - | Verma et al. 2021 |
| 15. Bengaluru | 112 | Below 20 | Cross-sectional | 48.00% | - | - | - | - | 5.40% | 4.50% | - | Anuradha and Manjunatha 2019 |
| 16. Maharashtra | 100 | 10–19 | Cross-sectional | 78.00% | - | - | - | - | - | 61.00% | - | Sharma et al. 2019 |
| 17. Purba Midnapore, West Bengal | 75 | 11–19 | Cross-sectional | 89.33% | - | - | - | - | - | 12.00% | - | Das et al. 2019 |
| 18. Nagpur | 600 | 10–19 | Cross sectional | 21.00% | - | - | 13.00% | 3.00% | - | 7.00% | 16.00% | Singh and Kasturwar 2017 |
| 19. North 24 Parganas, West Bengal | 280 | 14–19 | Cross-sectional | 51.07% | 92.14% | 59.64% | - | - | - | - | - | Sanyal and Ray 2008 |
| 20. Hyderabad | 120 | 11–16 | Cross-sectional | - | 28.00% | - | - | - | - | - | - | Kiran and Yashoda 2020 |
| 21. Haldwani | 297 | 10–19 | Cross-sectional | - | 19.53% | - | - | - | - | - | - | Goyal 2018 |
| 22. Himachal Pradesh | 111 | - | Cross-sectional | - | 19.80% | - | - | - | - | - | - | Walia et al. 2015 |
| 23. Ludhiana | 958 | 10–19 | Cross sectional | 12.80% | - | - | 6.70% | 1.10% | 1.90% | - | - | Kohli and Kapoor 2021 |
| 24. Maharashtra | 122 | 13–19 | Cross-sectional | 65.60% | 9.01% | - | - | - | - | - | - | Aggarwal et al. 2021 |
| 25. Patna, Bihar | 300 | 13–17 | Cross-sectional | 74.70% | 17.70% | - | - | - | - | - | - | Singh et al. 2023 |
| 26. Ludhiana, Punjab | 50 | 13–18 | Cross-sectional | 62.00% | - | - | - | - | - | - | Dharani and Sood 2018 | |
| 27. Maharashtra | 620 | 10–19 | Cross-sectional | 44.20% | 16.90% | - | - | - | - | - | - | Patil et al. 2009 |
| 28. Wardha | 300 | 10–19 | Cross-sectional | 67.00% | - | - | - | - | - | - | - | Mudey et al. 2010 |
| 29. Kolkata, West Bengal | 325 | 10–19 | Cross-sectional | 15.08% | 95.38% | 33.85% | - | - | - | - | - | Ray et al. 2010 |
| 30. Davanagar, Karnataka | 200 | 10–19 | Cross-sectional | 56.50% | - | - | - | - | - | - | - | Basavaraju et al. 2019 |
| 31. Bangalore | 190 | 12–17 | Cross-sectional | 52.60% | - | - | - | - | - | - | - | Kailashraj et al. 2020 |
| 32. Odissa | 250 | 10–19 | Cross-sectional | 28.00% | - | - | 27,00% | - | - | - | 10.00% | Behera et al. 2017 |
| 33. Wardha | 200 | 12–16 | Cross-sectional | - | - | 47.00% | - | - | - | - | Nimbhorkar et al. 2023 | |
| 34. Marathewara, Maharashtra | 122 | 13–19 | Cross-sectional | 54.10% | 32.79% | - | - | - | - | - | - | Fatima et al. 2023 |
| 35. Prayagraj | 500 | 15–19 | Cross sectional | 57.00% | 16.00% | - | - | - | - | - | - | Saxena et al. 2023 |
| 36. Kerala | 461 | 10–19 | Cross-sectional | - | 36.22% | - | - | - | - | - | - | Geroge and Sabita 2019 |
| 37. Raichur | 80 | 13–16 | Cross sectional | 47.50% | 16.30% | - | - | - | - | - | - | Ade and Patil 2013 |
| 38. Jaipur | 180 | 10–19 | Cross-sectional | 41.66% | 13.33% | - | - | - | - | 25.00% | - | Yadav and Masand 2018 |
| 39. Nellore | 169 | 11–16 | Cross-sectional | 71.60% | 8.90% | - | - | - | - | - | - | Chinta et al. 2018 |
| 40. Karnataka | 430 | 12–16 | Cross-sectional | 56.50% | 33.02% | 17.4% | - | - | - | - | - | Mann and Ts. 2023 |
| 41. Bhopal, Madhya Pradesh | 350 | 10–17 | Cross-sectional | - | 42.30% | - | - | - | - | - | - | Mekle et al. 2020 |
| 42. Maharashtra | 323 | 15–19 | Cross-sectional | 18.30% | 5.60% | - | - | - | - | - | - | Kanotra et al. 2013 |
| 43. Kheda, Gujarat | 200 | 13–18 | Cross-sectional | 62.00% | 30.50% | - | - | - | - | - | - | Prajapati et al. 2015 |
| 44. Sabarkanta, Gujarat | 250 | 13–18 | Cross sectional | - | 37.20% | - | - | - | - | - | - | Aggarwal et al. 2017 |
| 45. Mangaluru, Karnataka | 132 | 13–18 | Cross-sectional | - | 30.32% | - | - | - | - | - | - | Senapathi and Kumar 2018 |
| 46. West Bengal | 86 | Below 20 | Cross-sectional | 48.3% | - | - | - | - | - | - | - | Lalbiaknungi et al. 2015 |
| 47. Kamrup, Assam | 350 | 10–19 | Cross-sectional | 88.30%* | 9.10% | - | - | - | - | - | - | Majhi and Das 2020 |
*Combined paper (1)
**Mild, moderate, and severe dysmenorrhoea was combined
Abbreviations: PMS: Premenstrual Syndromes
| Study area | Study sample | Studied age group (in years) | Study design | Menstrual disorders | Reference | |||||||
| Dysmenorrhoea | Irregular menstruation | PMS | oligomenorrhea | Polymenorrhea | Amenorrhea | Menorrhagia | hypomenorrhea | |||||
| 1. Nagpur | 290 | 10–19 | Cross sectional | - | 14.14% | - | - | - | - | - | - | Borkar et al. 2022 |
| 2. Balasore, Odissa | 450 | 10–19 | Cross sectional | 28.88% | 25.10% | 79.11% | - | - | - | 24.44% | 90.00% | Mahapatra 2023 |
| 3. Achampet Mandal | 425 | 10–19 | Cross-sectional | 25.00% | - | 21.51% | - | 18.98% | - | - | - | Sridhar and Gauthami 2017 |
| 4. Khunti, Jharkhand | 150 | 13–18 | Cross-sectional | - | 30.66% | - | - | - | - | - | - | Kumari et al. 2021 |
| 5. Chittor, Andhra Pradesh | 293 | 10–19 | Cross-sectional | - | 17.70% | - | - | - | - | - | - | Udayar et al. 2016 |
| 6. Mumbai | 114 | 11–18 | Cross-sectional | 70.18% | - | - | - | - | - | - | Meshram et al. 2020 | |
| 7. Amravati | 150 | Below 20 | Cross-sectional, descriptive, and comparative | - | 12.60% | - | - | - | - | - | - | Kale et al. 2023 |
| 8. Bhubaneswar, Odissa | 300 | 10–16 | Cross-sectional | 52.00% | 23.91% | - | - | - | - | - | - | Jena et al. 2017 |
| 9. West Garo hills of Meghalaya | 100 | 13–18 | Cross-sectional | 97.00% | - | - | - | - | - | - | - | Nagar and Aimol 2010 |
| 10. Tamil Nadu | 507 | 12–18 | Cross-sectional | 78.30% | 32.30% | - | - | - | - | - | - | Shanmugananth et al. 2023 |
| 11. Jalpaiguri, West Bengal | 301 | 10–19 | Cross-sectional | 78.07% | 30.89% | 89.70% | - | - | - | - | - | Thakur et al. 2020 |
| 12. Garo Hills of Meghalaya | 240 | 12–18 | Cross-sectional | 62.5% | 44.2% | - | - | - | - | - | - | Nagar et al. 2022* |
| 13. Jammu and Kashmir | 131 | 13–15 | Cross-sectional | - | 18.320% | - | - | - | - | - | - | Dhingra et al. 2009 |
| 14. Maharashtra | 277 | 12–16 | Cross-sectional | 15.80% | 23.40% | - | - | 14.80% | 11.50% | - | Kakeri et al. 2018 | |
*Combined paper (2)
Abbreviations: PMS: Premenstrual Syndromes
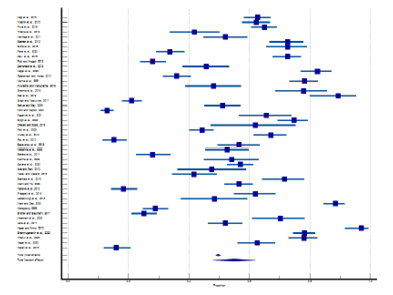
Figure 3. Forest plot of meta-analysis of proportion of dysmenorrhea
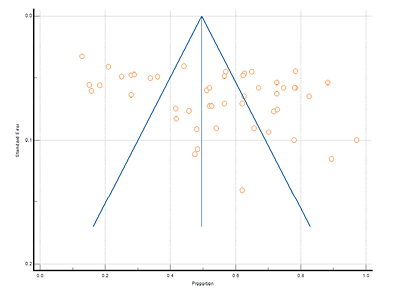
Figure 4. Funnel plot of meta-analysis proportion of dysmenorrhea
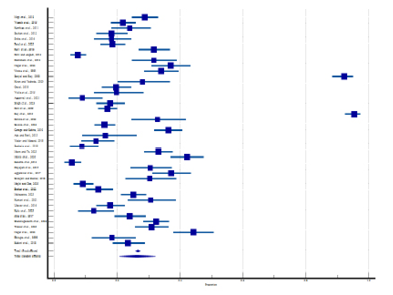
Figure 5. Forest plot of meta-analysis of the proportion of irregular menstruation
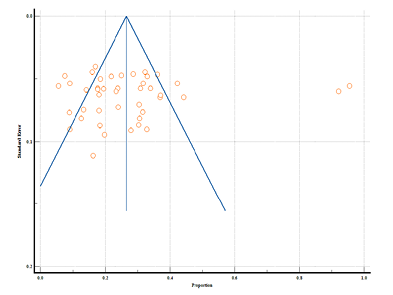
Figure 6. Funnel plot of meta-analysis proportion of irregular menstruation
Figure 7 shows the forest plot of 11 studies that reported PMS. Overall results were represented through the diamond at the bottom with a 95% confidence interval. The highest prevalence of PMS in rural areas was found to be at 65.50% (95% CI: 57.86 to 72.59) by Dambhare et al. (2012), and the lowest prevalence was found at 17.40% (95% CI: 13.93 to 21.32) by Mann and Ts. (2023). Only 3 studies were solely limited to tribal areas, among them the highest prevalence of PMS was found at 89.70% (95% CI: 85.69 to 92.89) by Thakur et al. (2020), while the lowest prevalence was found at 21.51% (95% CI: 17.69 to 25.72) by Sridhar and Gauthami (2017). The random effect model showed overall prevalence was 47.49% (95% CI: 31.44 to 63.81) and indicated a significant heterogeneity (Q-1105.30, DF-10, p<0.0001, I2-99.10%). Figure 8 represents the funnel plot that indicates no publication bias was found by Egger’s test (p=0.637).
Figure 9 depicts 6 studies reporting oligomenorrhea among rural areas. This disorder was not found in reported studies among tribal areas. The diamond at the bottom represents the overall results with a 95% confidence interval. The graph indicates that the highest prevalence of oligomenorrhea was found at 27.00% (95% CI: 21.59 to 32.95) by Behera et al. (2017) and the lowest prevalence was found at 6.70% (95% CI: 5.20 to 8.47) in a study by Kohli and Kapoor (2021). The random effect model of pooled prevalence was 13.88% (95% CI: 8.98 to 19.65). This estimate indicates a significant heterogeneity (Q-89.34, DF-5, p<0.001, I2- 94.40%). Egger’s test showed no evidence of publication bias by funnel plot in Figure 10 (p= 0.056).
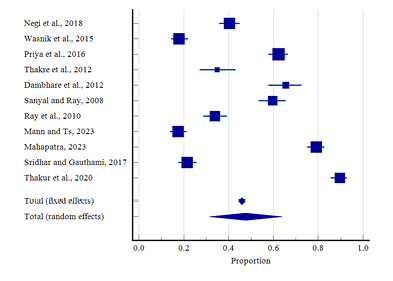
Figure 7. Forest plot of meta-analysis of proportion of PMS
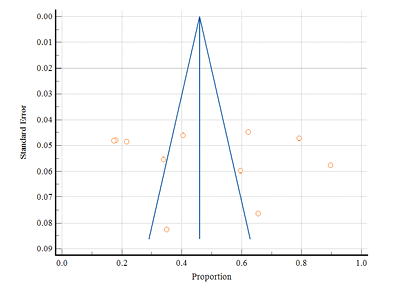
Figure 8. Funnel plot of meta-analysis proportion of PMS
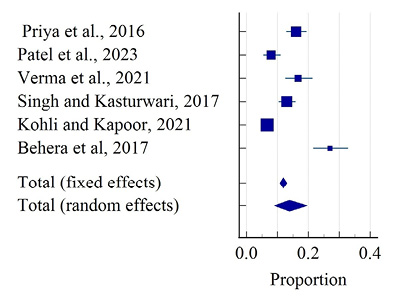
Figure 9. Forest plot of meta-analysis of the proportion of oligomenorrhea
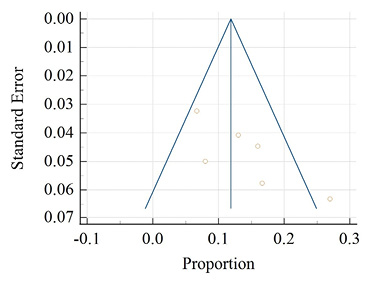
Figure 10. Funnel plot of meta-analysis proportion of oligomenorrhea
Figure 11 depicts a total of 7 studies that reported polymenorrhea, with only 2 belonging to tribal areas. Overall, results with a 95% confidence interval were represented through the diamond at the bottom. The highest prevalence in rural areas was found at 24.75% (95% CI: 20.59 to 29.28) by Patel et al. (2023), and the lowest prevalence was found at 0.45% (95% CI: 0.05 to 1.62) in a study by Patil and Angadi (2013). In tribal areas the highest prevalence of polymenorrhea was found at 18.98% (95% CI:15.36 to 23.03) by Sridhar and Gauthami (2017), and the lowest prevalence was found at 14.80% (96% CI: 10.83 to 19.53) by Kakeri et al. (2018). The overall prevalence of polymenorrhea was 7.85% (95% CI: 2.30 to 16.31) estimated by the random effect model which showed a significant heterogeneity (Q-356.45, DF-6, P<0.0001, I2-98.32%). There was no evidence of publication bias by Egger’s test (p=0.130; Fig. 12).
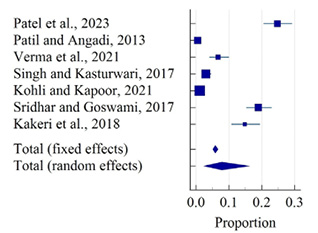
Figure 11. Forest plot of meta-analysis of proportion of polymenorrhea

Figure 12. Funnel plot of meta-analysis proportion of polymenorrhea
Figure 13 shows a total of 12 studies indicating menorrhagia with only 2 belonging to tribal areas. The diamond at the bottom represents the overall results with a 95% confidence interval. The highest prevalence in rural areas was found at 61.00% (95% CI: 50.73 to70.59) in a study by Sharma et al. (2019), and the lowest prevalence was found at 4.50% (95% CI: 1.55 to 9.90) by Anuradha and Manjunatha (2019). In tribal areas the highest prevalence of menorrhagia was found at 24.40% (95% CI:20.53 to 28.68) among adolescent girls of various tribal communities by Mahapatra (2023), while the lowest prevalence was found at 11.50% (95% CI: 7.99 to 15.85) by Kakeri et al. (2018). The random effect model showed the overall prevalence of menorrhagia at 16.83% (95% CI: 10.04 to 24.96). A significant heterogeneity was present (Q-431.03, DF-11, P<0.0001, I2-97.45%). There was no evidence of publication bias by Egger’s test (p=0.321; Fig. 14).
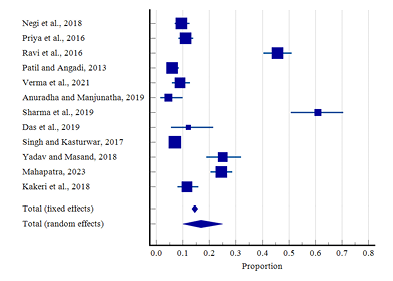
Figure 13. Forest plot of meta-analysis of proportion of menorrhagia
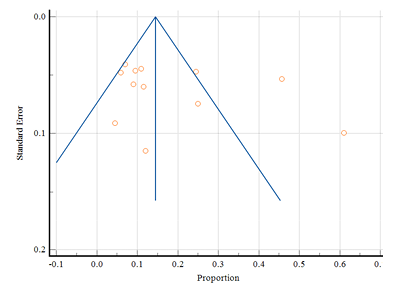
Figure 14. Funnel plot of meta-analysis proportion of menorrhagia
Table 4 shows that, among 61 reviewed articles, only 17 (12 from rural and 5 from tribal areas) reported associated factors with menstrual disorders among adolescent girls. Table 4 shows major associated factors of menstrual disorders were lack of physical activities, dietary habits, BMI, socio socioeconomic factors among rural and tribal adolescent girls in India.
| Rural areas | |||||
| Study area | Study sample | Studied age group (in years) | Study design | Associated factors | Reference |
| 1. Garhwal | 470 | 13–19 | Cross-sectional | Junk food Lack of physical activity |
Negi et al. 2018 |
| 2. Tamil Nadu | 500 | 14–19 | Cross-sectional | BMI | Priya et al. 2016 |
| 3. Lucknow | 254 | 10–19 | Cross-sectional | Age | Sachan et al. 2012 |
| 4. Haryana | 300 | 17–19 | Cross-sectional | Nutritional status Dietary habits Socio-economic factors |
Verma et al. 2021 |
| 5. Maharashtra | 100 | 10–19 | Cross-sectional | Age factors of the participants | Sharma et al. 2019 |
| 6. Nagpur | 600 | 10–19 | Cross-sectional | Age of the adolescents Education of mothers Education of the respondents BMI |
Singh and Kasturwar 2017 |
| 7. North 24 Parganas, West Bengal | 280 | 14–19 | Cross-sectional | Age groups Socio-economic factors Occupation and education of parents |
Sanyal and Ray 2008 |
| 8. Ludhiana | 958 | 10–19 | Cross-sectional | Age factors | Kohli and Kapoor 2021 |
| 9. Kolkata, West Bengal | 325 | 10–19 | Cross-sectional | Socio economic variables | Ray et al. 2010 |
| 10. Kerala | 461 | 10–19 | Cross-sectional | Age factors | Geroge and Sabita 2019 |
| 11. Karnataka | 430 | 12–16 | Cross-sectional | Clinico-Socio-demographic factors | Mann and Ts 2023 |
| 12. West Bengal | 86 | Below 20 | Cross-sectional | Socio-economic group BMI |
Lalbiaknungi et al. 2015 |
| Tribal areas | |||||
| 13. Bhubaneswar, Odessa | 300 | 10–16 | Cross-sectional | Socio-economic factors | Jena et al. 2017 |
| 14. West Garo hills of Meghalaya | 100 | 13–18 | Cross-sectional | Age of the respondents Total family income |
Nagar and Aimol 2010 |
| 15. Tamil Nadu | 507 | 12–18 | Cross-sectional | Lack of knowledge and awareness Cultural taboos and restrictions Poor nutrition and socio-economic factors |
Shanmugananth et al. 2023 |
| 16. Jalpaiguri, West Bengal | 301 | 10–19 | Cross-sectional | BMI Educational status of participants Skipping the menstrual cycle |
Thakur et al. 2020 |
| 17. Jammu and Kashmir | 131 | 13–15 | Cross-sectional | Low level of knowledge regarding menstruation Mother’s attitude towards menstruation |
Dhingra et al. 2009 |
Abbreviations: BMI: Body Mass Index
The present review systematically summarizes the prevalence of menstrual disorders among adolescent girls living in rural and tribal areas in India. In rural areas, the highest prevalence of dysmenorrhea (89.33%) was found in Purba Midnapore, West Bengal by Das et al. (2019). This finding are in line with a questionnaire-based study conducted among 757 Malaysian adolescent girls, showing 85.7% of adolescents experienced dysmenorrhea out of which 42.1% have moderate dysmenorrhea and 11.2% have severe dysmenorrhea (Azhary et al. 2022). 88.0% of adolescent girls were experiencing dysmenorrhea in Portugal (Marques et al. 2022). 80.4% of Swedish adolescents were experiencing dysmenorrhea (Gambadauro et al. 2024). Irregular menstruation (95.38%) was found highest in Kolkata, West Bengal by Ray et al. (2010). A study conducted among 106 rural Nepali found that 76.6% of adolescent girls were having irregular periods (Chhetri and Singh 2020). Whereas the lowest prevalence of oligomenorrhea (6.70%) and amenorrhea (1.90%) was found in Ludhiana by Kohli and Kapoor (2021). These results were quite similar to the survey reported by Aryani et al. (2018) conducted among 444 Indonesian adolescent girls, which showed that 24.5% of the girls experienced oligomenorrhea, polymenorrhea was found among 5.9% of girls and only 0.2% of the girls were experiencing amenorrhea. On the contrary, the highest prevalence of oligomenorrhea (27.00%) and the lowest prevalence of hypomenorrhea (10.00%) were found in Odisha (Behera et al. 2017). Another significant finding includes Amenorrhea which was found the highest (5.40%) and menorrhagia was found the lowest (4.50%) in Bengaluru (Anuradha and Manjunatha 2019). PMS (Pre-menstrual syndromes) was higher (65.50%) in Wardha, Maharashtra (Dambhare et al. 2012), and the lowest prevalence (17.40%) was reported in Karnataka by Mann and Ts (2023). In Sweden, 1100 adolescent girls had at least one menstrual problem reported as either moderate (81.3%) or severe (31.3%) followed by mood swings (81.1%) (Gambadauro et al. 2024). The lowest prevalence of dysmenorrhea (15.80%), polymenorrhea (14.80%), and menorrhagia (11.50%) were reported in Maharashtra (Kakeri et al. 2018). On the contrary, the highest prevalence of menorrhagia (24.44%) was reported among adolescent girls from various tribal communities (Mahapatra 2023). The highest prevalence of dysmenorrhea (78.30%) and irregular menstruation (44.2%) was found in the West Garo hills of Meghalaya (Nagar and Aimol 2010; Nagar et al. 2022). The lowest prevalence of PMS (21.50%) and the highest prevalence of polymenorrhea (18.90%) were reported in Achampet Mandal, Andhra Pradesh (Sridhar and Gauthami 2017). In addition, the highest prevalence of PMS (89.70%) was found among the adolescents of Jalpaiguri district of West Bengal (Thakur et al. 2020). A study conducted by Sharma et al. (2016) in the Pokhara Valley of Nepal, showed that 64.2% of the girls had irregular menstruation followed by oligomenorrhea (23.1%). Another study conducted in Northwest Ethiopia reported that 75.4% of the adolescents were experiencing PMS (Zegeye et al. 2009). The prevalence of oligomenorrhea and amenorrhea was not found in these studies.
This review reveals an overall prevalence of dysmenorrhea, irregular menstruation, PMS, oligomenorrhea, polymenorrhea, and menorrhagia among adolescent girls in rural and tribal areas and the meta-analysis shows the prevalence was 54.96% (95% CI: 47.93 to 61.85), 26.21% (95% CI: 20.73 to 32.09), 47.49% (95% CI: 31.44 to 63.81), 13.88% (95% CI: 8.98 to 19.65),7.85% (95% CI: 2.30 to 16.31), 16.83% (95% CI: 10.04 to 24.96) respectively. Similar results were reported in the review done by Samani et al. (2018) among Iranian adolescent girls, showing the overall pooled prevalence of dysmenorrhea, oligomenorrhea, polymenorrhea, and menorrhagia was 73.27% (95% CI: 65.12 to 81.42), 13.11% (95% CI: 10.04 to 16.19), 9.94% (95% CI: 7.33 to 12.56%) and 19.24% (95% CI: 12.78 to 25.69). Prevalence of PMS and irregular menstruation was not reported in the review. Based on a large Italian data, 6.7% (95% CI: 5.4% to 7.0%) and 9.0% (95% CI: 7.7% to 9.4%), 3.0% (95% CI: 2.5% to 3.4%) of adolescent girls were suffering from dysmenorrhea, irregular menstruation, and polymenorrhea, oligomenorrhea, and menorrhagia was found among 3.4% (95% CI: 2.9% to 3.9%) and 19.0% (95%CI: 17.9% to 20.1%) of adolescent girls respectively (Rigon et al. 2012).
This review also tried to highlight as much as possible about the factors associated with menstrual disorders. Dysmenorrhea and irregular menstruation were associated with food habits while PMS was associated with a lack of physical activities (Negi et al. 2018) which can lead to school absenteeism (Priya et al. 2016). Considerable pain during menstruation affects daily activities among adolescent girls in Southwestern Nigeria (Amu and Bamidele 2014). School absenteeism was also due to the menstrual pain among adolescent girls in Nepal (Sharma et al. 2016). BMI was found to have a strong association with these disorders (Jena et al. 2017; Priya et al. 2016; Singh and Kasturwar, 2017; Thakur et al. 2020; Verma et al. 2021) and similar results were reported by Bahadori et al. (2023) among Iranian adolescent girls. The age of the respondents plays an important role in menstrual-related problems (Sanyal and Ray 2008; Nagar and Aimol 2010; Singh and Kasturwar 2017; George and Sabita 2019; Sharma et al. 2019; Kohli and Kapoor 2021). Late adolescents were more likely to have menstrual irregularities (Singh and Kasturwar 2017). The age of the respondents was found to have a significant association with menstrual disorders among adolescent girls in other countries as well (Marques et al. 2022; Paudel 2022). For example, studies found that socioeconomic factor is another significant factor associated with various menstrual disorders (Sanyal and Ray 2008; Ray et al. 2010; Nagar and Aimol 2010; Lalbiaknungi et al. 2015; Jena et al. 2017; Verma et al. 2021; Shanmugananth et al. 2023). Socioeconomic status was also associated with menstrual disorders in Nepal and Sweden (Chhetri and Singh, 2020; Gambadauro et al. 2024). Lack of knowledge about menstruation was associated with menstrual irregularities among Gujjar tribal adolescent girls (Dhingra et al. 2009). Another study among tribal adolescent girls in Tamil Nadu found that low socio-economic status, lack of knowledge, and poor nutrition can be responsible for the high prevalence of primary dysmenorrhea, and Cultural restrictions and taboos play a major role during menstruation. Adolescents often hesitate to talk about their menstruation-related problems due to cultural restrictions and taboos and, as a result, the ability to bear menstrual pain in girls increases (Shanmugananth et al. 2023). Mothers often consider menstruation as a negligible issue, for that reason most mothers are found to be reticent to their daughters regarding menstruation and its related issues (Dhingra et al. 2009). Similarly, mother’s education was found to have a strong connection among adolescent girls in Nepal and has major restrictions on religious or family activities (Chhetri and Singh 2020). The previous study found that PMS was associated with some clinico-socio-demographic factors, such as age, class, menstrual cycle regularity and duration, and menstrual flow which results in increasing depression and anxiety among them (Mann and Ts 2023). Similar results were reported among Iranian adolescent girls (Bahadori et al. 2023).
Meta-analysis with small studies (<5) can lead to narrow confidence intervals and estimation of heterogeneity is quite difficult in this situation which can result in a biased effect (Mathes and Kuss 2018). Hence, two disorders (amenorrhea and hypomenorrhea) were exempted from meta-analysis due to unavailability of more than 5 articles. Only 3 articles were found to have these two disorders. This study is limited to English-language publications. Authors have tried as much as possible to read all the relevant articles that indicate menstrual disorders among adolescent girls in rural and tribal areas in India. Some relevant articles might have been missed because it is humanly impossible to read all the articles.
This study shows the overall prevalence of menstrual disorders i.e., dysmenorrhea, irregular menstruation, PMS, oligomenorrhea, polymenorrhea, and menorrhagia among adolescent girls in rural and tribal areas in India. This review reveals that the most predominant disorders were dysmenorrhea, irregular menstruation along menorrhagia in both areas. Adolescents refused treatment due to shame and discomfort. Lack of physical activities, dietary habits, BMI, socio socioeconomic factors were linked to menstrual irregularities among adolescent girls in India. The study also revealed that adolescent girls in both areas at least have one problem related to menstruation and the prevalence of these menstrual disorders varies in different areas all over India. Adolescent girls in rural and tribal areas in India were in a vulnerable situation during menstruation.
Future scope
This review is one of the first attempts to study menstrual disorders among adolescent girls specifically in rural and tribal areas in India. With this review, we have tried to provide information related to the overall prevalence of menstrual disorders and associated factors among them in both areas. However, future research needs to be done on this specific aspect. Awareness should be taken at home as well as in schools. Proper guidance on every aspect of menstruation should be arranged before and after attained menarche. Due to low socio-economic status, many girls may not have access to healthcare or any treatment, so health camps should be organized in different rural as well as tribal areas.
Abbreviations
BMC – BioMed Central. CASP – Critical Appraisal Skills Programme, PRISMA- Preferred Reporting Items for Systematic Reviews and Meta-Analysis, PROSPERO – International Prospective Register of Systematic Reviews, UNICEF – United Nations Children’s Emergency Fund, WHO – World Health Organization.
Acknowledgement
Authors are grateful to all the authors whose articles have been reviewed and discussed and to all the adolescent girls who participated in their study.
Funding
This study is supported by Savitribai Jyotirao Phule fellowship for single girl child (Grant no: UGCES-22-GE-WES-F-SJSGC-9520) provided by the University Grants Commission (UGC). The authors do not receive any grant for publication specifically for this research work.
Conflict of interest
none
Author’s contribution
SC gave the idea. RC conceptualized and designed the whole study. Registration of the review, literature reviews, quality assessment of studies, data analysis was done by RC and then discussed with SC. RC wrote the whole manuscript and checked it by SC. All authors have read, checked, and approved the whole manuscript.
Ade A, Patil R. 2013. Menstrual hygiene and practices of rural adolescent girls of Raichur. Int J Biol Med Res 4(2):3014–3017.
Aggarwal S, Ambalkar D, Madhumathi J, Badge V, Humne A. 2021. Menstrual Hygiene Practices of Adolescent Girls in Rural Maharashtra. Indian J. Gend. Stud 28(1): 127–137. doi: https://doi.org/10.1177/0971521520974879
Agarwal V, Fancy MJ, Shah H, Agarwal AS, Shah JH, Singh S. 2017. Menstrual Hygiene: Knowledge and Practice among Adolescent Girls of Rural Sabarkantha District. Natl J Community Med 8(10):597–601.
Amu EO, Bamidele JO. 2014. Prevalence of menstrual disorders among adolescent girls in Osogbo, South Western Nigeria. Int J Adolesc Med Health 26(1):101–106. doi: 10.1515/ijamh-2013-0500. PMID: 24501153.
Anuradha RV, Manjunatha S. 2019. A Study on Menstrual Problems among High School Girls Studying in Rural Field Practice Area of a Tertiary Care Hospital. J Med Sci 5(3):70–72. doi: https://doi.org/10.5005/jp-journals-10045-00127
Aryani I, Rachma U, Rokhayati E, Moelyo A. 2018. Menstrual cycle patterns of Indonesian adolescents. Paediatrica Indonesiana 58(3):101.
Azhary JMK, Leng LK, Razali N, et al. 2022. The prevalence of menstrual disorders and premenstrual syndrome among adolescent girls living in North Borneo, Malaysia: a questionnaire-based study. BMC Womens Health 22(1):341.
Bahadori F, Sahebazzamani Z, Ghasemzadeh S, Kousehlou Z, Zarei L, Hoseinpour M. 2023. Menstrual Cycle Disorders and their Relationship with Body Mass Index (BMI) in Adolescent Girls. J. Obstet. Gynecol. Cancer Res 8(4): 327–334. doi: 10.30699/jogcr.8.4.327
Basavaraju V, Bukanakere CL, Dsouza MJ, Mallenahalli AKS. 2019. A study of menstrual hygiene among rural adolescent school girls in rural field practice area of a medical college, Davangere- a cross-sectional study. Int J Community Med Public Health 6(10):4538–4544.
Behera JC, Sathpathy U, Das L. 2017. Burden of Menstrual Disorders in Adolescent Girls: A Comparative Study Among Rural and Urban Population. Sch. J. App. Med. Sci 5(6D):2359–2364. doi: 10.36347/sjams.2017.v05i06.058
Borkar SK, Borkar A, Shaikh MK, Mendhe H, Ambad R, Joshi A. 2022. Study of Menstrual Hygiene Practices Among Adolescent Girls in a Tribal Area of Central India. Cureus 14(10):e30247. doi: 10.7759/cureus.30247. PMID: 36381734; PMCID: PMC9652700.
Chhetri DD, Singh MS. 2020. Menstrual Characteristics among the Nepali Adolescent Girls. Indian J Public Health Res Dev 11(7): 247–253.
Chinta K, Sasikala P, Chandrasekhar V, Jayanth C, Geethanjali A. 2018. Menstrual hygiene patterns and practices among rural adolescent school girls. Int J Community Med Public Health 5:5190–5194. doi: http://dx.doi.org/10.18203/2394-6040.ijcmph20184788
Critical Appraisal Skills Programme. 2024. CASP (systematic review) Checklist. Available at: https://casp-uk.net/checklists/casp-systematic-review-checklist.pdf. Assessed 15 May 2024.
Dambhare DG, Wagh SV, Dudhe JY. 2012. Age at menarche and menstrual cycle pattern among school adolescent girls in Central India. Glob J Health Sci 4(1):105–111. doi: 10.5539/gjhs.v4n1p105. PMID: 22980118; PMCID: PMC4777020
Das S, Thakur J, Goswami M. 2019. Menstrual Health and Hygiene Practices among the Rural and Urban Adolescents of West Bengal, India. Anthropol Ethnol Open Acc J 2(1):000114.
DerSimonian R, Laird N. 1986. Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. PMID: 3802833.
Dharni I & Sood AK. 2018. Menstrual Hygiene Practices Among Adolescent Girls of Urban and Rural Schools of Ludhiana, Punjab. Nursing Journal of India CIX 178–183. doi:10.48029/NJI.2018.CIX403
Dhingra R, Kumar A, Kour M. 2009. Knowledge and practices related to menstruation among tribal (Gujjar) adolescent girls. Ethno Med 3:43–48.
Fatima M, Kausar H, Giri P, Ingale A. 2023. Study on Menstrual Hygiene Management among Urban and Rural Adolescent Girls in Marathwada Region of Maharashtra, India. Indian J. of Com. Health 35(2):146–151. doi: 10.47203/IJCH.2023.v35i02.003
Gambadauro P, Hadlaczky G, Wasserman D, Carli V. 2023. Menstrual symptoms and subjective well-being among postmenarchal adolescents. AJOG Glob Rep 4(1):100304. doi: 10.1016/j.xagr.2023.100304. PMID: 38304304; PMCID: PMC10830861.
George L, Sabitha N. 2019. Prevalence of Menstrual Disorders and Menstrual Hygiene among School Going Adolescent Girls in Central Kerala. FoodSci: Indian Journal of Research in Food Science and Nutrition 6(2):13–18. doi:10.15613/fijrfn/2019/v6i2/190524
Goyal N. 2018. Pattern and features of menstruation among adolescent girls in Haldwani: a cross-sectional study. Int J Reprod Contracept Obstet Gynecol 7:2805–2812.
Jena P, Andalib S, Khuntia S, Mishra A. 2017. Spectrum of menstrual disorder and health consciousness of adolescent school going girls: A comparative study between the extremes of two socio-economic groups. Indian J Obstet Gynecol Res 4(3):235–239.
Kailasraj KH, Basavaraju V, Kumar J, Manjunatha S. 2020. A study of knowledge and practice of menstrual hygiene among adolescent school girls in rural and urban field practice area of RajaRajeswari Medical College and Hospital, Bangalore, India. Int J Community Med Public Health 7(2):665–672. doi: http://dx.doi.org/10.18203/2394-6040.ijcmph20200446
Kakeri M, Patil SB, Waghmare R. 2018. Knowledge and practice gap for menstrual hygiene among adolescent school girls of Tribal district of Maharashtra, India: a cross sectional study. Ind J Youth Adol Health 5(3):23–27. doi:10.24321/2349.2880.201816
Kale PV, Warbhe PA, Jawarkar AK, Khanande VD. 2023. Menstrual Related Practices and Hygiene Among Adolescent Girls-A Comparative Study from Private, Government and Tribal Schools of Amravati District. Int J Med Res Health Sci 12(2):48–55.
Kanotra SK, Bangal VB, Bhavthankar DP. 2013. Menstrual pattern and problems among rural adolescent girls. Int J Biomed Adv Res 4(8):551–554. doi:10.7439/ijbar.v4i8.426
Karthiga V, Boratne AV, Datta SS, Joice S, Abraham SB, Purty AJ. 2011. Menstrual problems and pattern of consultation among adolescent school girls in Pondicherry. Indian J Med Spec 2(2):92–95.
Kiran KV, Yashoda K. 2020. Menstrual Hygiene Management among Rural Adolescent Girls of Agrarian Families. Int.J.Curr.Microbiol.App.Sci 9(01):891–899. doi: https://doi.org/10.20546/ijcmas.2020.901.099
Kohli I, Kapoor P. 2021. Medical and psychosocial health status of adolescent school going girls. Int. J. Paediatrics Geriatrics 5(1):19–24. doi: https://doi.org/10.33545/26643685.2022.v5.i1a.156
Kumari S, Sood S, Davis S, Chaudhury S. 2021. Knowledge and practices related to menstruation among tribal adolescent girls. Ind Psychiatry J 30(Suppl 1):S160–S165. doi: 10.4103/0972-6748.328808. Epub 2021 Oct 22. PMID: 34908683; PMCID: PMC8611595.
Kumbhar SK, Reddy M, Sujana B, Reddy K R, Bhargavi K D, Balkrishna C. 2011. Prevalence Of Dysmenorrhea Among Adolescent Girls (14–19 Yrs) Of Kadapa District and Its Impact on Quality of Life: A Cross Sectional Study. Natl J Community Med 2(02):265–268.
Lalbiaknungi L, Roy S, Paul A, Dukpa R. 2015. A study on menstrual hygiene and dysmenorrhea of adolescent girls in a rural population of West Bengal. J. Compr. Health 3:33–41.
Majeed J, Sharma P, Ajmera P, Dalal K. 2022. Menstrual hygiene practices and associated factors among Indian adolescent girls: a meta-analysis. Reprod Health 19(1):148. doi: 10.1186/s12978-022-01453-3. PMID: 35739585; PMCID: PMC9229495.
Majhi TK, Das A. 2020. A Study on Problems of Menstruation among Adolescent Girls and Its Related Cultural Practices as Expressed by Mother in Rural Community of Kamrup District Assam. Int. J. Adv. Sci. Res 4(3):6–11.
Mahapatra T. 2023. Menstrual health and status of tribal adolescent girls of Balasore, Odisha. International Journal of Science and Research Archive 08(01):393–403. doi: https://doi.org/10.30574/ijsra.2023.8.1.0067
Mann P, Ts P. 2023. Premenstrual Syndrome, Anxiety, and Depression Among Menstruating Rural Adolescent Girls: A Community-Based Cross-Sectional Study. Cureus 15(12):e50385. doi: 10.7759/cureus.50385. PMID: 38213363; PMCID: PMC10783120.
Marques P, Madeira T, Gama A. 2022. Menstrual cycle among adolescents: girls’ awareness and influence of age at menarche and overweight. Rev Paul Pediatr 40:e2020494. doi: 10.1590/1984-0462/2022/40/2020494. PMID: 35019010; PMCID: PMC8734600
Mathes T, Kuss O. 2018. A comparison of methods for meta-analysis of a small number of studies with binary outcomes. Res Synth Methods 9(3):366–381. doi: 10.1002/jrsm.1296. PMID: 29573180.
Mekle D, Rathore R, Dixit J, Kapoor A. 2020. Perception and practices pertaining to menstruation among adolescent girls. Int J Pediatr Res 7(3):146–151. doi:10.17511/ijpr.2020.i03.06
Meshram P, Ratta A, Kumar V. 2020. Perceptions and practices related to menstruation amongst tribal adolescent girls in rural field practice area of tertiary health care institute in Mumbai. Int J Community Med Public Health 7(4). doi:10.18203/2394-6040.ijcmph20201034
Mudey AB, Keshwani N, Mudey GA, Goyal RC. 2010. A cross sectional study on the awareness regarding safe and hygienic practices amongst school going adolescent girls in the rural areas of Wardha district. Glob. J. Health Sci 2(2):225–231. doi:10.5539/gjhs.v2n2p225.
Nagar S, Aimol KH. 2010. Knowledge of adolescent girls regarding menstruation in tribal areas of Meghalaya. Stud Tribes Tribals 8:27–30.
Negi P, Mishra A, Lakhera P. 2018. Menstrual abnormalities and their association with lifestyle pattern in adolescent girls of Garhwal, India. J Family Med Prim Care 7(4):804–808. doi: 10.4103/jfmpc.jfmpc_159_17. PMID: 30234057; PMCID: PMC6132013.
Nimbhorkar SP, Jumade PP, Rahate NP. 2023. Knowledge, Perceptions, Taboos, and Practices of Menstrual Hygiene among Adolescent Girls in Urban and Rural Areas of Central India. J South Asian Feder Obst Gynae 15(6):696–702. doi: https://doi.org/10.5005/jp-journals-10006-2344
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. 2021. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. doi: 10.1136/bmj.n71.
Patel S, Patel B, Pushpalatha K, et al. 2023. Health care seeking behaviour for common menstrual problems and their determinants in Adolescent girls at rural setting in Central India: A mix-method study. medRxiv 2023. doi: 10.1101/2023.08.01.23293531
Patil MS, Angadi MM. 2013. Menstrual pattern among adolescent girls in rural area of Bijapur. Al Ameen J Med Sci 6(1):17–20.
Patil SN, Wasnik V, Wadke R. 2009. Health problems amongst adolescent girls in rural areas of Ratnagiri district of Maharashtra. J Clin Diagnostic Research 3(5):1784–1790.
Poudel P. 2022. Pattern of menstruation and its problem among adolescent girls: a school based cross-sectional study. Int J Contemp Pediatr 9:635–640.
Pearlstein T, Steiner M. 2008. Premenstrual dysphoric disorder: Burden of illness and treatment update. J Psychiatry Neurosci 33:291–301.
Petticrew M, Roberts H. 2008. Systematic reviews in the social sciences: A practical guide. John Wiley & Sons.
Prajapati D, Shah J, Kedia G. 2015. Menstrual Hygiene: Knowledge and Practice among Adolescent Girls of Rural Kheda District. Natl J Community Med 6(3): 349–353.
Priya SS, Alliratnam A, Shankar RA. 2016. Menstrual problems and hygiene among rural adolescent girls of Tamil Nadu– A cross sectional study. Indian J Obstet Gynecol Res 3(2):126–131.
Rajaretnam T, Hallad JS. 2010. Menarche, menstrual problems and reproductive tract infections among adolescents in the rural and urban areas of northern Karnataka in India. European population Conference 1–4 September.
Ravi R, Shah P, Palani G, Edward S, Sathiyasekaran BW. 2016. Prevalence of Menstrual Problems among Adolescent School Girls in Rural Tamil Nadu. J Pediatr Adolesc Gynecol 29(6):571–576. doi: 10.1016/j.jpag.2015.10.016. Epub 2015 Oct 29. PMID: 26537316.
Ray S, Mishra SK, Roy AG, Das BM. 2010. Menstrual characteristics: a study of the adolescents of rural and urban West Bengal, India. Ann Hum Biol 37(5): 668–681. doi: 10.3109/03014460903563442. PMID: 20166852.
Rigon F, De Sanctis V, Bernasconi S, Bianchin L, Bona G, Bozzola M. et al. 2012. Menstrual pattern and menstrual disorders among adolescents: An update of the Italian data. Ital J Pediatr 38:38.
Sachan B, Idris MZ, Jain S, Kumari R, Singh A. 2012. Age at menarche and menstrual problems among school-going adolescent girls of a north indian district. Journal of Basic and Clinical Reproductive Sciences 1(1):56–59.
Samani RO, Hashiani AA, Razavi M, Vesali S, Rezaeinejad M, Maroufizadeh S, Sepidarkish M. 2018. The prevalence of menstrual disorders in Iran: A systematic review and meta-analysis. Int J Reprod Biomed 16(11):665–678. PMID: 30775681; PMCID: PMC6350848.
Sanyal S, Ray S. 2008. Variation in the menstrual characteristics in adolescents of West Bengal. Singapore Med J 49(7):542–550. PMID: 18695862.
Saxena S, Gupta V, Shweta K. 2023. Knowledge, attitude and practices regarding reproductive health among rural and urban adolescent girls. Int J Reprod Contracept Obstet Gynecol 12(1):83–87. doi: https://dx.doi.org/10.18203/2320-1770.ijrcog20223254
Senapathi P, Kumar H.A. 2018. Comparative study of menstrual hygiene management among rural and urban adolescent girls in Mangaluru, Karnataka. Int J Community Med Public Health 5(6):2548–2556. doi: http://dx.doi.org/10.18203/2394-6040.ijcmph20182193
Shanmugananth E, Ghose S, Vinod S, Hemalatha R, Hariharan S. 2023. Prevalence of Primary Dysmenorrhea among School-Going Girls in Tribal Populations in Tamilnadu, India. Adv. Biores 14(4):164–171. doi: 10.15515/abr.0976-4585.14.4.164171
Sharma P, Malhotra C, Taneja DK, Saha R. 2008. Problems related to menstruation amongst adolescent girls. Indian J Pediatr 75(2):125–129. doi: 10.1007/s12098-008-0018-5. PMID: 18334791.
Sharma S, Deuja S, Saha CG. 2016. Menstrual pattern among adolescent girls of Pokhara Valley: a cross sectional study. BMC Womens Health 16(1):74. doi: 10.1186/s12905-016-0354-y. PMID: 27938370; PMCID: PMC5148896.
Sharma S, Joshi R, Korake R. 2019. Study on menstrual cycle of adolescent girls of rural area of Ambajogai Tehsil of Beed district. J Pharm Innov 9(1):331–334.
Singh D, Kasturwar NB. 2017. Pattern of menstrual morbidities and the associated socio-demographic factors among adolescent girls in a rural area of Nagpur. Panacea J Med Sci 7(2):77–82.
Singh G, Gupta A, Anand N, Kumar R. 2023. Determinants of menstrual hygiene among adolescent school girls in a rural area of Patna, Bihar, India: A cross-sectional study. J Family Med Prim Care 12(12):3271–3278. doi: 10.4103/jfmpc.jfmpc_891_23. Epub 2023 Dec 1. PMID: 38361840; PMCID: PMC10866283.
Sinha S, Srivastava J, Sachan B, Singh R. 2016. A study of menstrual pattern and prevalence of dysmenorrhea during menstruation among school going adolescent girls in Lucknow district, Uttar Pradesh, India. Int J Community Med Public Health 3(5):1200–1203. doi: http://dx.doi.org/10.18203/2394-6040.ijcmph20161384
Sridhar D, Gauthami N. 2017. Menstrual health status and cultural practices of tribal adolescent girls. Int J Community Med Public Health 4(11):4120–4124. doi: http://dx.doi.org/10.18203/2394-6040.ijcmph20174648
Thakur J, Goswami M, Roy S. 2020. Understanding menstrual characteristics from the perspective of reproductive energetics: a study on the adolescent Oraon tribal populations. Anthropological Review 83(2):109–128. doi: 10.2478/anre-2020-0009.
Thakre SB, Thakre SS, Ughade S, Thakre AD. 2012. Urban-Rural Differences in Menstrual Problems and Practices of Girl Students in Nagpur, India. Indian Pediatrics 49(9): 433–436.
Udayar SE, Kruthika K, Devi PV. 2016. Menstrual Hygiene Practices Among Adolescent Girls Residing in Tribal and Social Welfare Hostel In Andhra Pradesh: A Community Based Study. Natl J Community Med 7(08):681–685.
Uman LS. 2011. Systematic reviews and meta-analyses. J Can Acad Child Adolesc Psychiatry 20(1):57–59. PMID: 21286370; PMCID: PMC3024725.
UNICEF. 2018. FAST FACTS: Nine things you didn’t know about menstruation. Available at: https://www.unicef.org/press-releases/fast-facts-nine-things-you-didnt-know-about-menstruation. Assessed 4 May 2024.
Verma V, Das B, Nath J. 2021. Determination of the prevalence and pattern of menstrual disorders in college going adolescent girls in rural Haryana. Int J Reprod Contracept Obstet Gynecol 10(7):2729–2733.
Wasnik VR, Dhumale D, Jawarkar AK. 2015. A study of the menstrual pattern and problems among rural school going adolescent girls of Amravati district of Maharashtra, India. Int J Res Med Sci 3(5):1252–1256. doi: 10.5455/2320-6012.ijrms20150539
Walia DK, Yadav RJ, Pandey A, Bakshi RK. 2015. Menstrual patterns among school going adolescent girls in Chandigarh and rural areas of Himachal Pradesh, North India. Natl J Community Med 6:583–586.
WHO. 2022. WHO statement on menstrual health and rights. Available at: https://www.who.int/news/item/22-06-2022-who-statement-on-menstrual-health-and-rights. Assessed 2 May 2024.
Yadav A, Masand D L F. 2018. Study of menstrual disorder in adolescent girls at tertiary care centre in rural area. Int J Reprod Contracept Obstet Gynecol 7(5):1979–1983. doi: http://dx.doi.org/10.18203/2320-1770.ijrcog20181941
Zegeye DT, Megabiaw B, Mulu A. 2009. Age at menarche and the menstrual pattern of secondary school adolescents in northwest Ethiopia. BMC Womens Health 9:29. doi: 10.1186/1472-6874-9-29. PMID: 19804623; PMCID: PMC2763859.

