Available online at: https://doi.org/10.18778/1898-6773.87.1.08
 https://orcid.org/0000-0002-1458-783X
https://orcid.org/0000-0002-1458-783X
Institute of Archaeology and Ethnography, National Academy of Science, Republic of Armenia
 https://orcid.org/0000-0002-7898-5693
https://orcid.org/0000-0002-7898-5693
Department of Medical Biology, Yerevan Mkhitar Heratsi State Medical University, Yerevan, Republic of Armenia
 https://orcid.org/0000-0003-0191-5395
https://orcid.org/0000-0003-0191-5395
Institute of Archaeology and Ethnography, National Academy of Science, Republic of Armenia
 https://orcid.org/0000-0003-3019-8145
https://orcid.org/0000-0003-3019-8145
Institute of Archaeology and Ethnography, National Academy of Science, Republic of Armenia
 https://orcid.org/0000-0002-7875-828X
https://orcid.org/0000-0002-7875-828X
Institute of Archaeology and Ethnography, National Academy of Science, Republic of Armenia
 https://orcid.org/0000-0003-3400-8759
https://orcid.org/0000-0003-3400-8759
Department of Forensic Medicine, Yerevan Mkhitar Heratsi State Medical University, Yerevan, Republic of Armenia
 https://orcid.org/0009-0004-3331-2577
https://orcid.org/0009-0004-3331-2577
Institute of Applied Problems of Physics of National Academy of Science, Republic of Armenia
 https://orcid.org/0000-0001-6323-3790
https://orcid.org/0000-0001-6323-3790
Institute of Applied Problems of Physics of National Academy of Science, Republic of Armenia
ABSTRACT: The skeleton in question derives from the Late Iron Age monument of Nor Armavir and was unearthed from burial No. 19. The deceased was buried in an unusual position. In this article, we characterize the pathological bony changes indicative of tuberculous spondylitis. The skeleton was subject to a detailed macroscopic investigation. Besides age at death estimation and sex determination, a careful palaeopathological evaluation was performed on the bone remains. In addition, volumetric (3D) computed tomography was carried out on four lumbar vertebrae (L2–5) to complement the macromorphology-based diagnosis.
KEY WORDS: Armenia, Late Iron Age, Bioarcheology, Paleopathology, Tuberculous Spondylitis.
Tuberculous spondylitis is the most common form of bone and joints tuberculosis (An and Seldomridge 2006; Karadimas et al. 2008). The disease was already mentioned by Hippocrates and Galen (Turunc et al. 2007; Gouliouris et al. 2010) but the first detailed description of its main symptoms (hump and associated paralysis) was given in 1779 by the English surgeon Percivell Pott, after whom the disease was named (Pott’s disease).
Tuberculous of the spine, or tuberculous spondylitis, is an infectious disease caused by Micobacterium tuberculosis and Micobacterium bovis, which cause clinically indistinguishable from each other diseases (Torres-Gonzalez et al. 2016). Molecular analysis of DNA has been carried out on archaeological samples and no evidence of Micobacterium bovis has been found (Zink et al. 2007). Tuberculous of the spine is characterized by the formation of specific granulomas and the progressive destruction of bone, leading to pronounced organic and functional disorders of the affected part of the skeleton (Pereira and Lynch 2005). The thoracic spine (60%) is the most common site of lesions, followed by the lumbar spine (30%). The cervical spine (5%) and the sacral spine (5%) are affected to a lesser extent (Mushkin et al. 2012; Ratobylsky et al. 2012). The number of vertebral bodies affected varies widely. Vertebral bodies of 2–3 vertebral bodies are most commonly found in first-time cases (65% of cases), and destruction of a single vertebral body is found in 1–3% of cases (Kotze and Erasmus 2006; Huang et al. 2009; Barinov and Malchenko 2013). The incidence of tuberculous spondylitis is higher in men than in women (Burrill et al. 2007; Cottle and Riordan 2008).

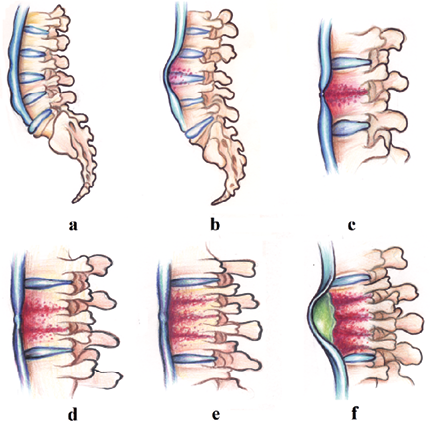
Fig. 1. Schematic representation of the development of tuberculous spondylitis: a) unaltered spine, b) lesion of two adjacent vertebral bodies, c) reduced height of the affected vertebral bodies and involvement of the intervertebral disc, d) lesion of the third adjacent vertebral body, e) lesion of the fourth adjacent vertebral body, f) formation of a paravertebral abscess under the anterior longitudinal ligament and irreversible wedge-shaped deformation of the affected vertebrae at several levels
Tuberculous spondylitis occurs as a result of reactivation and transfer of the source of infection from the primary complex, which may be located in the lungs or in another organ (Diachenko 1958). Tuberculous inflammation develops in the vertebral bodies. Initial foci occur in the marginal regions of the vertebral bodies close to the intervertebral discs. Mycobacteria may ‘nest’ in the bone marrow even though the bone tissue is not anatomically or clinically altered (Dyachenko 1958; Nather et al. 2005; Lee et al. 2015). In the bone system (including the spine), the onset of the local pathological process is expressed by a reactive inflammatory process around the pathogen and the development of an infectious granuloma. As the inflammation progresses, areas of necrosis appear.
The tuberculous nidus may develop in the central parts of the vertebral body-central type of vertebral lesion with a small involvement of intervertebral discs, but more often, the process is localized in the adjacent parts of the vertebral bodies and in the intervertebral disc-intervertebral type of tuberculous spondylitis. The intradiscal route is characterized by the primary involvement of the cartilaginous intervertebral disc with the hyaline boundary lamina. The boundary lamina acts as a buffer, but in adults it is weaker and less homogeneous than in children, which favours the growth of a tuberculous granuloma from the primary focus, which may spread vertically. This subchondral spread of the process disrupts the bone-cartilage junction, affecting the nutrition of the lamina and reducing its stability. As a result, the cartilaginous lamina degenerates, elements of tubercle tissue sprout through it, and the process moves to the nucleus pulposus and through it to the second lamina and the adjacent vertebra. The observed decrease in the height of the intervertebral cartilage is one of the early signs of spondylitis and depends primarily on a decrease in the elasticity of all its elements, especially the nucleus pulposus, which, when it germinates or perforates the cartilaginous lamina, eventually loses its dense covering, spreads and softens. The changes in the intervertebral disc are predominantly necrotic and curdled (Kornev 1953).
In the extradiscal route from the primary focus, granulomas are observed to sprout through the cortical layer of the vertebral body in its anterior, lateral or posterior regions. Most often, the primary focus is located closer to the anterior surface, which, on the one hand, leads to the weakening of the anterior stability of the vertebral body and its wedge-shaped deformity and, on the other hand, to the spread of the process to the anterior or lateral surface of the body under or through the periosteum, with the subsequent formation of “superfusion”. If the granuloma occurs to the lateral surface of the vertebra not covered by the anterior longitudinal ligament, the abscess forms close to the vertebra–paravertebral, first under the periosteum and then beyond it. In such cases, the abscess (unilateral or bilateral) may remain in situ and take on a globular “swallow’s nest” shape. In those anatomical areas where muscles attach to vertebral bodies, abscesses may spread and migrate through the intermuscular spaces (Kornev 1953).
The changes described above lead to marked destruction of the vertebrae. The anterior vertebral body is compressed and becomes wedge-shaped, forming a typical angular hump (Fig. 1).
In this work, we have applied a modern method of radial diagnostics, which allows us to obtain a volumetric (3D) image of the pathology of the spine of a Late Iron Age individual from the Nor Armavir monument. Archaeological excavations headed by S.A. Hmayakyan (with the participation of archaeologists N.G. Tiratsyan, M.S. Hmayakyan) were carried out in the territory of the Nor Armavir monument (Armavir Province) in 2022. Armavir Province (western part of Armenia) is located in the Ararat Plain, dominated by Mount Ararat to the south and Mount Aragats to the north; the province’s capital is the city of Armavir (Fig. 2). The province shares a 72 km border with Turkey to the south and west. The territory of Armavir has been inhabited since the 5th millennium BC. The ancient Urartian settlement of Argishtikhinili (Nor Armavir) was founded in 776 BC by King Argishti I. One of the oldest written records of the region was found in the inscriptions of the Urartian king Rusa II (685–645 BC). The area was one of the most important regions of ancient Armenia since the Urartu period. Nor Armavir is a village in the Armavir province of Armenia. The Nor Armavir necropolis includes primary burials and cremations; the latter are represented by burnt bones while flesh, sometimes of multiple individuals (Khudaverdyan et al. 2022).
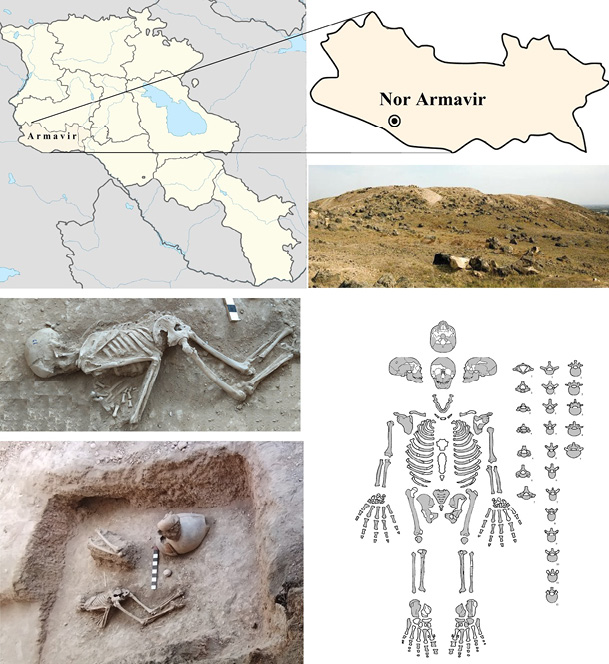
Fig. 2. Map showing the location of Armavir province (Armenia) where of Nor Armavir monument is located. Burial 19 (skeleton location). Diagrammatic illustration of the skeleton of No. 19. Missing parts are intentionally left blank; preserved ones were marked in gray
Evidence from the skeletal remains of these inhabitants of Armenia can add to our understanding of their lives. Here, we describe the remains of an adult skeleton with spinal anomalies. The study of human biological material from archaeological contexts is in a key position to facilitate cross-disciplinary research, to understand the past and present populations through ancient remains, and to make substantial theoretical contributions to the broader social sciences (Larsen 1997; Buikstra and Beck 2006).
Burial 19 from Nor Armavir monument contained a partially complete but well-preserved (grade 1 according to McKinley et al. 2004) skeleton of an individual (Fig. 2). The remains in burial no. 19 were lying in an unnaturally bent position with the arms and head clearly pulled down and the head turned to the west. The lumbar spine and hips were elevated. A small bronze necklace was found around his neck.
Sex and age at death were estimated using standard anthropological methods. Sex was determined based on cranial and pelvic morphology (Phenice 1969; Buikstra and Ubelaker 1994). Age at death was determined based on the degree of obliteration of the cranial sutures (Meindl and Lovejoy 1985; Buikstra and Ubelaker 1994) and dental wear (Cox and Mays 2000; AlQahtani et al. 2010). The stature was reconstructed on the basis of the long bones after Trotter and Gleser (1958).
3D computed microtomography (MicroCT) was used at the Institute of Applied Problems of Physics of NAS RA to assess the condition of intervertebral discs. Computed tomography allowed high-resolution (55 micrometers) observation and analysis of the condition of the intervertebral discs, which was simultaneously used to generate virtual 3D reconstructions. This method allows for conducting a detailed topographic analysis of the texture of the vertebral different slices, edge morphology, fracture angles and bone deformation.
The remains from the burial belonged to a man aged 40–49 years (Fig. 2). The metric variables and the values measured on the hip bones are shown in Table 1. Pathological lesions are located exclusively in the spine (Fig. 3), particularly in the lumbar region. He was found to have a severe pathology – fusion of several lumbar vertebrae (L2–L5). The vertebrae of this segment had been deformed and fused together during the individual’s lifetime. The bodies show both osteoblastic and osteoclastic activity (Fig. 3).
| Right | Left | ||
| Humerus | |||
| 1 | Maximal length | 326 | 331 |
| 2 | Total length | 322 | 329 |
| 3 | Upper epiphysis breadth | – | 53.2 |
| 4 | Maximal midshaft breadth | 63.2 | 64.2 |
| 5 | Largest diameter Ø of the middle diaphysis | 23.8 | 24.6 |
| 6 | Smallest Ø of the middle diaphysis | 20 | 19.9 |
| 7 | Minimal midshaft breadth | 65 | 65.5 |
| 7a | Midshaft circumference | 70 | 72 |
| 7:1 | Robusticity index | 19.94 | 19.8 |
| 6:5 | Cross-section index | 84.1 | 80.9 |
| Radius | |||
| 1 | Maximal length | 253 | 252 |
| 2 | Physiological length | 242 | 242 |
| 4 | Cross-section diameter | 12.2 | 12.8 |
| 5 | Sagittal shaft diameter | 16 | 14 |
| 3 | Minimal shaft circumference | 40 | 39.5 |
| 3:2 | Robusticity index | 16.6 | 16.4 |
| 5:4 | Cross-section index | 131.2 | 109.4 |
| Ulna | |||
| 1 | Maximal length | 277 | – |
| 2 | Physiological length | 243 | 238 |
| 11 | Sagittal diameter | 14.7 | 13.8 |
| 12 | Transverse diameter | 16.5 | 18 |
| 13 | Upper transverse diameter | 21.6 | 21.7 |
| 14 | Upper sagittal diameter | 24 | 26.8 |
| 3 | Minimal shaft circumference | 36.5 | 36 |
| 3:2 | Robusticity index | 15.1 | 15.2 |
| 11:12 | Cross-section index | 89.1 | 76.7 |
| 13:14 | Platyleny index | 90 | 80.98 |
| Femur | |||
| 1 | Maximal length | – | 461 |
| 2 | Natural length | – | 452 |
| 21 | Condylar breadth | – | 85 |
| 6 | Sagittal diameter of midshaft | 31.7 | 32 |
| 7 | Transverse midshaft diameter | 27.8 | 28 |
| 9 | Upper transverse shaft diameter | 33 | 35.7 |
| 10 | Upper sagittal shaft diameter | 26.5 | 27.5 |
| 8 | Midshaft circumference | 93 | 95 |
| 8:2 | Robusticity index | – | 21.1 |
| 6:7 | Pilastry index | 114.1 | 114.3 |
| 10:9 | Platymery index | 80.4 | 77.1 |
| Tibia | |||
| 1 | Full length | – | 370 |
| 2 | Condylo-talar length | – | 350 |
| 1a | Maximal length | – | 371.5 |
| 5 | Upper epiphysis breadth | – | 83 |
| 6 | Lower epiphysis breadth | 51.5 | 49.4 |
| 8 | Sagittal diameter at midshaft level | 33 | 34 |
| 8a | Sagittal diameter at the nutrient foramen level | 39 | 41.3 |
| 9 | Transverse diameter at midshaft level | 20 | 21 |
| 9a | Transverse diameter at the nutrient foramen level | 23 | 25.5 |
| 10 | Midshaft circumference | 85 | 90 |
| 10b | Minimal shaft circumference | 77 | 77.5 |
| 9:8 | Cross-section index | 60.7 | 61.8 |
| 10b:1 | Robusticity index | – | 20.95 |
| 9a:8a | Cross-section index | 58.98 | 61.8 |
| 10:1 | Robusticity index | – | 24.4 |
| Fibula | |||
| 1 | Maximum length | – | 357 |
| Skeletal proportions | |||
| R1:H1 | Brachial index | 77.7 | 76.2 |
| T1:F2 | Tibio-femoral index | – | 81.9 |
| H1+R1/F1+T1 | Intermembral index | – | 70.2 |
| H1+R1/ F2+T1 | Intermembral index | – | 70.93 |
| H1:F2 | Humero-femoralindex | – | 73.3 |
| R1:T1 | Radio-tibial index | – | 68.2 |
| Body length | 166.3 | ||
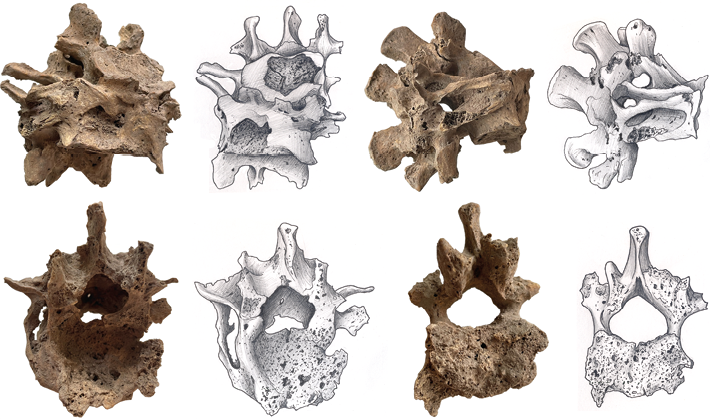
Fig. 3. Pathological lesions located in the spine. The destruction of the L2, L3, L4, and L5 bodies resulted in the sharp angular kyphosis in the upper lumbar region typical of the Pott’s disease
The intervertebral discs of the lumbar vertebrae (L2–L5) have lost their basic properties (elasticity and firmness) and have undergone decay. On direct projection images, the height of the intervertebral disc is reduced unevenly; in lateral projection, the discs are compressed anteriorly more than posteriorly, as the anterior parts of the cartilage are loaded and destroyed more than the posterior ones. There was involvement of adjacent vertebrae in the inflammatory process and formation of destruction of adjacent closure plates. The images show foci of destruction in the vertebral bodies (Fig. 3). During the destruction, the vertebral bodies were, to a large extent, destroyed and currently represent a large number of chaotically arranged dense bone fragments. The peripheral parts of the vertebral body are “eaten away” with sequestrum formation in caries-type destruction. This occurred as a result of the spread of the tuberculous granuloma under the ligamentous (under the anterior or posterior longitudinal ligament) from the primary destroyed vertebra to the neighboring vertebrae or through the disc in the area of the pulposus nucleus with subsequent destruction of the entire intervertebral disc.
The sequestrum has a rounded shape and looks like a “melting sugar cube” (Fig. 3). On the image, the sequestrum has a heterogeneous structure. The affected vertebral bodies wedge into each other, with the formation of an angular bend of the spinal axis with the apex pointing backwards (hump), which leads to irreversible deformation of the spine. The size of the hump depends on the number of vertebrae destroyed, and its presence is the most characteristic sign of tuberculous spondylitis. Defects in vertebral bodies are partially filled with newly formed bone trabeculae. Characteristic is irreversible deformation of the spine, pronounced degenerative-dystrophic changes in bone tissue.
Due to the application of technical features, the multi-slice X-ray tomography (3d) enables detecting all changes more clearly compared to standard radiography of the spine (Fig. 4). Figure 4 shows a cross-section of the spine with some adjacent vertebrae attached. The use of a MicroCT scan of the relevant area of the spine allowed us to look at the ankylosis. Upon external examination, the bodies of these vertebrae were not clearly visualized as part of the deformity, which is probably due to osteomalacia (destruction, fusion) of their larger part and further formation of secondary bone callus. MicroCT allowed us to detect the mass resulting from osteomalacia of the bodies of the 2nd, 3rd, 4th and 5th lumbar vertebrae, the nature of the fusion of the bodies and what was left of them. The pathology resulted in the formation of a bone mass approximately comparable in volume to the body of a single vertebra. The deformity was fused to the bone callus anteriorly. Bone layering, fine porosity and porosity are visible on the surface of the formation. There was probably a previous haemorrhage. Increased vascularisation of the bone promoted bone callus growth (Fig. 4). There appeared to be an inflammatory process as well.
Vertebral fusion resulted not only from osteomalacia of the bodies, but also from the overgrowth of the intervertebral joints and fusion of the arches. The spinous processes are practically intact due to the development of pathology, partially destroyed only when in burial. Interestingly, the ribs were still attached to the vertebrae. Anyway, there were upper and lower rib fossae on the vertebrae.
The upper (L2) and lower (L5) body surfaces are deformed, including at the edges, and there are signs of osteochondrosis and osteoporosis. A recessed area was formed in front of the bone conglomerate with a height of 26.2 mm, width of 23 mm, and depth of 12 mm. The surface itself is rough and porous (Fig. 3). Convergence of the spinous processes to the contact between the L4–L5 spinous processes is revealed. Convergence of the spinous processes is due to lumbar hyperlordosis, which inevitably occurs to compensate for kyphotic deformity and humping of the spine.
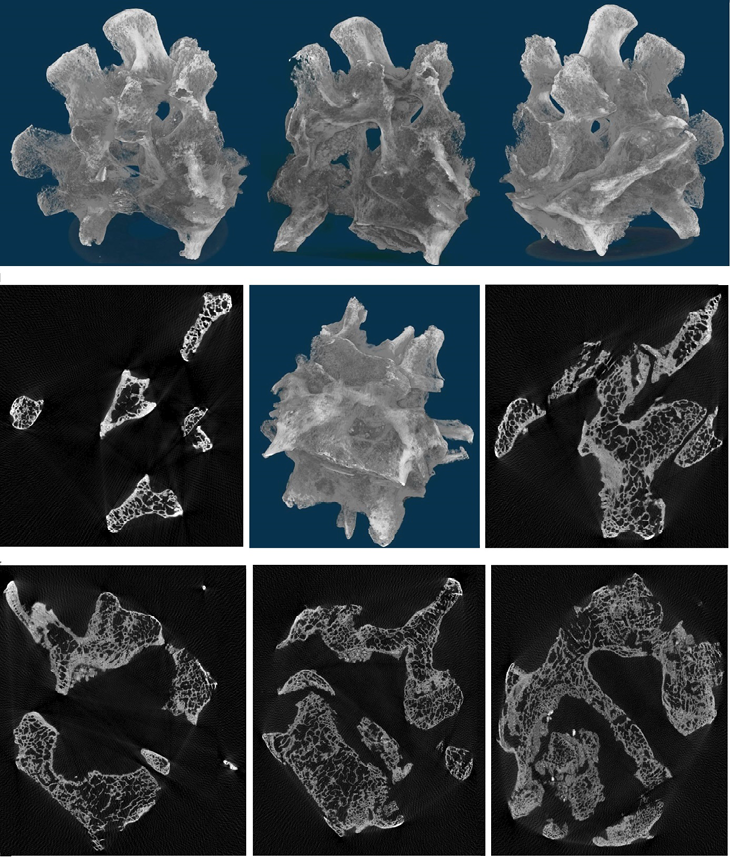
Fig. 4. Computed tomography of the fused vertebrae
The body length reconstructed from femur measurements calculated using Pearson and Lee’s formulae could be 166 cm. However, in terms of the deformity of the spine, the length of the body was rather unusual.
Tuberculous spondylitis accounts for about 40–50% of all bone tuberculosis cases. The thoracic vertebrae are affected most often (60%), while the lumbar vertebrae are less frequently affected (30%). The cervical region accounts for about 5–9%. The lower thoracic and upper lumbar regions are particularly susceptible to tuberculosis (Krasnobaev 1950; Kornev 1953; Talantov 1961; Mushkin et al. 2012; Ratobylsky et al. 2012). Men and people of young age are more prone to getting the disease. Among individuals with tuberculous spondylitis, 66% are children under 10 years of age, and 83.3% are individuals under 20 years of age (Kornev 1959; Gratsiansky and Khokhlov 1966).
The earliest case of tuberculous spondylitis dates back to the Neolithic (Bartels 1907; Baker et al. 2015, 2017). Palaeopathological lesions reminiscent of skeletal tuberculous spondylitis have been observed in northern Syria (Dja’de el Mughara, 8800–8300 BC). Typical lesions are found on the 9th and 10th thoracic vertebrae (Dja’de el Mughara). They are mainly lytic. The lower part of the 9th thoracic vertebra is completely destroyed, and the upper part of the 10th thoracic vertebra shows cystic rounded cavitations extending to the vertebral body, with a space-occupying mass aspect (Baker et al. 2015). In the site of Atlit Yam, the remains of an adult female and an immature individual presented paleopathological evidence of TB, confirmed by lipid biomarkers and aDNA analyses (Hershkovitz et al. 2008). In the same geographic area, paleopathological evidences of tuberculosis were previously mentioned for contemporaneous site (Ain Ghazal, ca. 7250 BC) (El-Najjar et al. 1996). In Egypt, in excavations of burials near the Nile River, 4 out of 10 skeletons (3000 BC) had signs of tuberculous spondylitis. E.G. Smith and W.R. Dawson (1924) provide a photograph of a mummy with tuberculous spondylitis and marked kyphosis of the thoracic spine. This individual lived in Egypt in the 11th to 10th century BC. D.G. Rokhlin and V.S. Maikova-Stroganova describe 5 cases of spinal tuberculosis in adults whose skeletons were found in Russia in burials from the last centuries BC and early AD (Rokhlin 1965; Galinskaya 2013). A skeleton of a man with tuberculous spondylitis was found in burial No. 2 of the Vaskovichi cemetery, Belarus (late 19th century) during the archaeological excavations (Vasiliev et al. 2022). The young man was lying on his back with his arms visibly slumped down and his spine unnaturally curved around his lower back. The case of subligamentous tuberculous spondylitis, a rare form of extrapulmonary tuberculosis, was diagnosed in the skeleton of a middle-aged man found in the ossuary of the Franciscan crypt of the church of Saints Anthony and Eusebius in north-western Italy (Larentis et al. 2020). The skeleton can be dated between the 17th and 19th centuries. Frequent cases of tuberculous spondylitis have been found in Armenia (Khudaverdyan 2005), Georgia (Pirpilashvili 1956), the Baltics (Darums 1970; Jankauskas 1998), Hungary (Nemeskery and Harsanyi 1959; Hlavenková et al. 2015), and Slovakia (Hanakova and Stloukal 1966; Kyselicová et al. 2015). The case of tuberculous spondylitis was present in Transylvania (Romania) during the 12th and 13th centuries. The paleopathological diagnosis was supported by M. tuberculosis analysis for complex ancient DNA (Hajdu et al. 2012).
Tuberculous spondylitis, found in an individual from Nor Armavir Monument, is a long, drawn-out disease that last for years. The rapidity of the development of this process may have depended on the local and general immunity of the individual, lack of treatment and physical stress on the spine. Due to the lack of any changes in the facet joints, it does not seem that vertebral collapse did occur, in spite of the important lytic destruction of the 4 vertebral bodies. This pattern is not commonly described in modern clinical practice but can be seen by medical imaging on living patients (Baba et al. 2013), and on skeletal materials (Pálfi et al. 2012; Baker et al. 2015). A typical sign of tuberculous spondylitis is deep, often subtotal contact destruction of the closure plates, which is an important differential diagnostic criterion. As we know, patients with tuberculous spondylitis stop walking because of pain, and with neurological insufficiency – because of weakness in the legs. They are characterized by sharp pain on percussion (latin percussio) of the spinous processes of the affected vertebrae, and on loading along the axis of the spine, there is the restriction of mobility in the damaged spine, its deformation. Under the skin, a thickening is formed, and then a bulge, the skin over which at first is not changed, but then as a result of the inflammatory process fuses with it, the abscess bursts outwards with the formation of a fistula, through which pus and necrotic masses are discharged. Individuals experience a change in gait: when walking, there is a slight leaning forward and towards the localization of the abscess. The development of the hump is caused by the destruction of vertebral bodies, accompanied by their flattening with wedge-shaped deformation of the destroyed vertebral bodies; the spinous process of the destroyed vertebrae protrudes most sharply and is located at the top of the hump, which has the shape of an angle. The size of the hump depends on the number of vertebrae destroyed, and its presence is the most characteristic sign of tuberculous spondylitis.
We have used diagnostic criteria derived from both the paleopathological and clinical literature.
А) The probable cause of this pathology was fractures of the bodies of the 2nd, 3rd, 4th and 5th lumbar vertebrae, fusion of the resulting bone mass, leading to a kyphosis bone conglomerate in the lumbar region. If there was trauma, which is the most likely scenario, the spinal cord and spinal nerves would have been damaged as well. In the case of trauma, this is determined by the migration of bone fragments into the spinal canal, canal occlusion, and in the case of purulent-inflammatory lesions – the development of an epidural abscess or myelitis (inflammation of the spinal cord). The formation of this pathology (resulting from a fracture) in the individual from Nor Armavir would be incompatible with life, primarily due to the formation of bone fragments and damage to the main vessels. Similarly, fractures caused by traumatic events rarely involve two or more vertebrae and, most importantly, they do not usually lead to osteoclastic activity, which is clearly evident in our individual case. In fact, osteoclastic activity is usually associated with the necrosis of isolated bone elements in a complex trauma, such as a comminuted fracture, which is not visible on a CT scan (Fig. 4). Because the processes leading to callus formation result in osteoblastic activity, the presence of mild and severe areas of lytic lesions in our individual allows us to easily rule out trauma as the cause of our findings (Lovell 1997).
B) Among inflammatory diseases, haematogenous osteomyelitis shares the greatest similarity to tuberculous spondylitis of the spine (Musaev 2000; Tikhodeev and Vishnevsky 2004; Nene and Bhojraj 2005). The most common causative agent of osteomyelitis is Staphylococcus aureus. In most cases, non-specific spondylitis is formed due to the haematogenous spread of the pathogen from the infectious focus, complicated by bacteremia. Common sources of infection include genitourinary tract, leg thrombophlebitis with chronic shin ulcers, pneumonia, bronchiectatic disease, streptoderma, furunculosis, tonsillitis, odontogenic infection (Mwachaka et al. 2011).
In addition to the haematogenous spread of infection, the venous route through the pelvic and vertebral plexus to the sacrum is well-known lumbar and lower thoracic vertebrae – for thrombophlebitis, paraproctitis, haemorrhoids, purulent processes in the pelvis and along the pharyngovertebral venous plexuses of the posterior surface of the pharynx to the upper and middle cervical vertebrae – in case of oral cavity infection, tonsillitis (Guseva et al. 2006). Pre-existing traumatic or dystrophic lesions of discs, joints, vertebral bodies, or processes may contribute to osteomyelitis at a particular level. That is, osteomyelitis of the spine is characterized by association with a past inflammatory disease, trauma, etc. (Ivanov et al. 2003). It is worth noting that sequestrations and abscesses in osteomyelitis are less common than in tuberculosis. Spinal deformity is less pronounced or absent in osteomyelitis. Unlike tuberculous spondylitis, osteomyelitis more often affects the posterior aspect of the vertebrae: the wishbones and transverse processes. Therefore, the diagnosis made for the individual from Nor Armavir is correct.
C) Syphilitic spondylitis results from the passage of syphilitic ulceration of the mucous membrane of the mouth and pharynx to the upper cervical vertebrae, where this lesion occurs predominantly. On radiological examination, bone sclerosis is typical. An individual from Nor Armavir has affected lumbar vertebrae.
D) Brucella spondylitis and tuberculous spondylitis, caused initially by bacteremia, are the two leading types of granulomatous spinal infections (Cordero and Sanchez 1991). Brucella spondylitis is easy to miss or may be misdiagnosed as tuberculous spondylitis. There was a significantly lower severity of vertebral destruction, vertebral posterior convex deformity, dead bone, and abscess scope in brucella spondylitis when compared to tuberculous spondylitis. Through the analysis of vertebra and intervertebral space, it was significantly lower in the severe vertebral destruction, vertebral posterior convex deformity, dead bone, and narrow – disappear change of intervertebral space in the patients with brucella spondylitis than those in tuberculous spondylitis (Yang et al. 2014; Liu et al. 2018; Guo et al. 2021).This widespread destruction in tuberculous spondylitis may result from the rapid involvement of the endplate (inflammatory reaction). With the progress of tuberculous spondylitis, gradually the vertebrae were severely destroyed. Our study found that the vertebral destruction in the individual from Nor Armavir was more severe. The vertebral erosion in tuberculous spondylitis was caseating granulomas and dead bone without new bone formation (Sapico and Montgomerie 1979).
E) Post-typhoid spondylitis usually occurs about a month after the end of typhoid fever and is localized most commonly in the lumbar spine. This disease is characterized by early, noticeable disc changes after only a few weeks. The intervertebral gap narrows and then completely disappears. The periosteum of the vertebral bodies is thickened and overgrown, so the lateral contours of the vertebrae also appear altered. It is not uncommon for bony brackets to form, fusing vertebral bodies and ossifying ligaments. The process is usually limited to 2 vertebrae and ends in bony ankylosis. In the person from Nor Armavir, post-typhoid spondylitis was also ruled out.
F) Actinomycosis of the spine is, as a rule, a secondary lesion and occurs when the fungus passes from the primary focus, localized in the submandibular region, in the lungs, oesophagus, intestine. Quite quickly, fistulas are formed, which are preceded by a dense (“like a board”) infiltrate against the background of this infiltrate and open the external fistula openings. The course of actinomycosis of the spine is long, resembling the course of tuberculous spondylitis with fistula. In radiological examination, focal destruction in the form of small patterns combined with sclerotic areas, moderate narrowing of the intervertebral gap corresponding to the lesion of the vertebral bodies, and development of ossification of ligaments in the form of “brackets” are typical. Spinal actinomycosis has also been ruled out in an individual from Nor Armavir.
G) Among the many non-tuberculous lesions, malformations, acquired deformities, neuralgia, lumbalgia, dystrophic and oncological diseases may be present. The malformations that may give rise to suspicion of tuberculous spondylitis are manifested mainly externally in the form of scoliosis, kyphoscoliosis and other spinal deformities associated with changes in the number and shape of the vertebrae and their relationship to each other and to other bones such as the skull, pelvis and ribs. These malformations are noticed in early childhood as soon as the child begins to sit up. In the beginning, they do not give any painful sensation, or any disturbance in the mobility of the spine, but later there is pain due to disturbance of the normal statics. Wedge-shaped vertebrae, or rather semi-vertebrae, are the most common developmental anomaly and cause of congenital scoliosis with a slight angular curvature, but without rotation around the axis (torsio), as in normal scoliosis. Non-inflammatory spinal lesions were also excluded in the individual from Nor Armavir.
H) Infectious arthritis is an acute or chronic inflammation that occurs in the joint bag due to bacterial or viral infection. The cause of arthritis is usually echinococcosis, which affects the bone tissue of the vertebrae, pelvic bones, and long bones of the limbs. Rheumatoid arthritis typically affects the periarticular area of the hands and joints (Grassi et al. 1998; Tesi et al. 2019). Reactive arthritis is characterized by peripheral arthritis, enthesopathy and asymmetric involvement of the sacroiliac joint, feet, heels, knees and ankles (Rogers and Waldron 1995; Carter and Hudson 2009). Psoriatic arthritis usually affects asymmetrically hands and feet, leading to a characteristic appearance of the disease in the phalanges of the hands and feet, known as “pencil-in-cup” (Rogers et al. 1987; Gladman 2005). Infectious arthritis, rheumatoid arthritis, reactive arthritis, psoriatic arthritis have also been excluded in the individual from Nor Armavir.
In this study, we presented a new probable case of tuberculous spondylitis. The pathology studied occurred long before the death of the individual who had been alive for years with that fused spine. Most probably, this deformity occurred because of chronic disease lasting for months or years, as evidenced by the vertebral fusion and bone callus formation. Such course of the disease is characteristic of tuberculous spondylitis. With such pathology of the spine, the man, of course, could not live a full life.
Acknowledgement
We are also grateful to the staff of the Institute of Archeology and Ethnography of the National Academy of Sciences of Armenia and Ani Sahakyan for a graphic reconstruction and illustration of bones and Tigranuhi Levonyan for scanning and processing of photos. We would like to thank all the reviewers for their detailed comments and suggestions on the manuscript.
Conflict of interest
The authors declare that there is no conflict of interest.
Authors’ contribution
The study was designed and conceived by AYK1. Fieldwork was carried out by SGH1, NGT1 and MSH1. 3D MicroCT was carried out by APA4 and VRK4. Analysis and interpretation were conducted by AYK1, AAY2, SAV3. The manuscript was written by AYK1 and VRK4.
AlQahtani SJ, Hector MP, Liversidge HM. 2010. Brief Communication: The London Atlas of Human Tooth Development and Eruption. Am J Phys Anthropol 42(3):481–90. https://doi.org/10.1002/ajpa.21258
An HS, Seldomridge JA. 2006. Spinal infections: diagnostic tests and imaging studies. Clin Orthop Relat Res 444:27–33. https://doi.org/10.1097/01.blo.0000203452.36522.97
Baba H, Tagami A, Adachi S, Hiura T, Osaki M. 2013. Tuberculosis affecting multiple vertebral bodies. Asian Spine J 7:222–6. https://doi.org/10.4184/asj.2013.7.3.222
Barinov VS, Malchenko OV. 2013. Extrapulmonary tuberculosis. Spec Lit: St. Petersburg.
Bartels P. 1907. Wirbelkaries in der jüngeren Steinzeit. Archiv für Anthropologie (N. F.) 6:233.
Brothwell DR. 1991. Digging up Bones. The Excavation, Treatment and Study of Human Skeletal Remains, third ed. Cornell University Press, Ithaca: New York.
Buikstra JE, Beck LA. 2006. Bioarchaeology: The Contextual Analysis of Human Remains. Academic Press: New York.
Buikstra JE, Ubelaker DH. 1994. Standards for Data Collection From Human Skeletal Remains. Arkansas Archaeological Survey: Fayetteville.
Burrill J, Williams C, Bain G, Conder G, Hine AL, Misra RR. 2007. Tuberculosis: a radiologic review. Radiographics 27(5):1255–1273. https://doi.org/10.1148/rg.275065176
Carter JD, Hudson AP. 2009. Reactive arthritis: Clinical aspects and medical management. Rheum Dis Clin North Am 35(1):21–44. https://doi.org/10.1016/j.rdc.2009.03.010
Cordero M, Sanchez I. 1991. Brucellar and tuberculous spondylitis. A comparative study of their clinical features. J Bone Joint Surg Br 73:100–103. https://doi.org/10.1302/0301-620X.73B1.1991738
Cottle L, Riordan T. 2008. Infectious spondylodiscitis. J Infect 56(6):401–412. https://doi.org/10.1016/j.jinf.2008.02.005
Cox M, Mays S. 2000. Human Osteology in Archaeology and Forensic Science. Cambridge: Cambridge University Press.
Darums VY. 1970. Diseases and healing in the ancient Baltics. Zinatne: Riga.
Dyachenko VA. 1958. X-ray diagnostics of diseases of bones and joints. Medgiz: Moscow.
El-Najjar M, Al-Sarie I, Al-Shiyab A. 1996. Cases of tuberculosis at ‘Ain Ghazal, Jordan. Paléorient 22:123–8. https://doi.org/10.3406/paleo.2003.4758
Galinskaya LA. 2013. Tuberculosis. Prevention and treatment. Phoenix: Rostov-on-Don.
Gladman DD. 2005. Psoriatic arthritis: Epidemiology, clinical features, course, and outcome. Ann Rheum Dis 64 (suppl_2):ii14–ii17. https://doi.org/10.1136/ard.2004.032482
Gouliouris T, Aliyu SH, Brown NM. 2010. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother 65(3):11–24. https://doi.org/10.1093/jac/dkq303
Grassi W, De Angelis R, Lamanna G, Cervini C. 1998. The clinical features of rheumatoid arthritis. Eur J Radiol 27:S18–S24. https://doi.org/10.1016/s0720-048x(98)00038-2
Gratsiansky VP, Khokhlov DK. 1966. Diagnosis of initial forms of osteoarticular tuberculosis. Medicine: Leningrad.
Guo H, Lan S, He Y, Tiheiran M, Liu W. 2021. Diferentiating brucella spondylitis from tuberculous spondylitis by the conventional MRI and MR T2 mapping: a prospective study. Eur J Med Res 26:125–132. https://doi.org/10.1186/s40001-021-00598-4
Guseva VN, Dolenko OV, Nekachalova AZ, Titarenko OT, Yakunova OA, Potapenko EI, Novikova NS. 2006. Clinical X-ray and laboratory features of tuberculosis and osteomyelitis of the spine. Probl Tuberk Bolezn Legk 11:9–13.
Hajdu T, Donoghue HD, Bernert Z, Fóthi E, Kővári I, Marcsik A. 2012. A case of spinal tuberculosis from the middle ages in Transylvania (Romania). Spine 37 (25):E1598–601. https://doi.org/10.1097/BRS.0b013e31827300dc
Hanáková H, Stloukal M. 1966. Staroslovanské pohfebiste v Josefove: Antropologicky rozbor. Academia: Praha.
Hershkovitz I, Donoghue HD, Minnikin DE, Besra GS, Lee OY, Gernaey AM, Galili E, Eshed V, Greenblatt CL, Lemma E, Bar-Gal GK, Spigelman M. 2008. Detection and molecular characterization of 9,000-year-old Mycobacterium tuberculosis from a Neolithic settlement in the Eastern Mediterranean. PLoS One 3:e3426. https://doi.org/10.1371/journal.pone.0003426
Hlavenková L, Teasdale MD, Gábor O, Nagy G, Beňuš R, Marcsik A, Pinhasi R, Hajdu T. 2015. Childhood bone tuberculosis from Roman Pécs, Hungary. Homo 66(1):27–37. https://doi.org/10.1016/j.jchb.2014.10.001
Huang QS, Zheng C, Hu Y, Yin X, Xu H, Zhang G, Wang Q. 2009. One-stage surgical management for children with spinal tuberculosis by anterior decompression and posterior instrumentation. Int Orthop 33(5):1385–1390. https://doi.org/10.1007/s00264-009-0758-5
Jankauskas R. 1998. History of human tuberculosis in Lithuania: possibilities and limitations of paleoosteological evidences. Bulletins et Mémoires de la Société d’anthropologie de Paris (Nouvelle Série) 10(3–4):357–374.
Karadimas EJ, Bunger C, Lindblad BE, Hansen ES, Høy K, Helmig P, Kannerup AS, Niedermann B. 2008. Spondylodiscitis. A retrospective study of 163 patients. Acta Orthop 79:650–659. https://doi.org/10.1080/17453670810016678
Khudaverdyan AYu. 2005. Atlas of paleopatological findings in the territory of Armenia. Van Aryan: Yerevan.
Khudaverdyan AYu, Hmayakyan SG, Tiratsyan NG, Hmayakyan MS. 2022. Paleoanthropology and Paleopatology of Bone Remains from the 7th Century BC Burials Found in the Nor Armavir Burial Ground (Armenia). Bulletin of the Moscow Region State University 5:115–141.
Kornev PG. 1953. Kostno-articular tuberculosis. Medgiz: Moscow.
Kotze D, Erasmus L. 2006. MRI findings in proven Mucobacterium tuberculosis (TB) spondylitis. SA Journal of Radiology 6:6–12.
Krasnobaev TP. 1950. Bone and joint tuberculosis in children. Medgiz: Moscow.
Kyselicová K, Šebest L, Beňuš R, Bognár C, Šarkan M, Dörnhöferová M. 2015. Skeletal manifestation of tuberculosis in the medieval population of Borovce (8th–12th century AD, Slovakia) in relationship to the occurrence of long bone changes and cribra orbitalia. Česká antropologie 65(2):16–22.
Larentis O, Tonina E, Tesi C, Rossetti C, Gorini I, Ciliberti R, Licata M. 2020. A probable case of subligamentous tuberculous spondylitis: The concealed body of the Late Modern Period (early 16th century to early 20th century), Franciscan crypt of St. Anthony and St. Eusebius church, Lombardy, Italy. Int J Osteoarchaeol 30:180–196. https://doi.org/10.1002/oa.2845
Larsen CS. 1997. Bioarchaeology: Interpreting Behavior from the Human Skeleton. Cambridge University Press, UK: Cambridge.
Lee KH, Goo JM, Lee SM, Park CM, Bahn YE, Kim H, Song YS, Hwang EJ. 2015. Digital Tomosynthesis for Evaluating Metastatic Lung Nodules: Nodule Visibility, Learning Curves, and Reading Times. Korean J Radiol 16(2):430–439. https://doi.org/10.3348/kjr.2015.16.2.430
Liu X, Li H, Jin C, Niu G, Guo B, Chen Y, Yang J. 2018. Diferentiation between brucellar and tuberculous spondylodiscitis in the acute and subacute stages by MRI: a retrospective observational study. Acad Radiol 25:1183–9. https://doi.org/10.1016/j.acra.2018.01.028
Lovejoy CO. 1985. Dental wear in the Libben population: its functional pattern and role in the determination of adult skeletal age at death. Am J Phys Anthropol 68:47–56. https://doi.org/10.1002/ajpa.1330680105
Lovell NC. 1997. Trauma analysis in paleopathology. Am J Phys Anthropol 105(25):139–170.
McKinley JI. 2004. Compiling a skeletal inventory: disarticulated and commingled remains. In: M Brickley, JI McKinley, editors. Guidelines to the Standards for Recording Human Remains. BABAO. 14–17.
Mwachaka PM, Ranketi SS, Nchafatso OG, Kasyoka BM, Kiboi JG. 2011. Spinal tuberculosis among human immunodeficiency virusnegative patients in a Kenyan tertiary hospital: a 5-year synopsis. Spine J 11(4):265–269. https://doi.org/10.1016/j.spinee.2011.01.033
Musaev ShM. 2000. Diagnosis and complex treatment of osteomyelitis of the spine. Dissertation of candidate of medical sciences. Kemerovo.
Mushkin AYu. 2006. Bone and joint tuberculosis in children: current situation and prognosis. Tuberculosis and lung diseases 11:13–16.
Nather A, David V, Hee HT, Thambiah J. 2005. Pyogenic vertebral osteomyelitis: a review of 14 cases. J Orthop Surg (Hong Kong) 13:240–244. https://doi.org/10.1177/230949900501300305
Nemeskéri J, Harsányi L. 1959. Die Bedeutung paläopathologischer Untersuchungen für die historische Anthropologie. Homo 10:203–226.
Nene A, Bhojraj S. 2005. Results of nonsurgical treatment of thoracic spinal tuberculosis in adults. Spine J 5:79–84. https://doi.org/10.1016/j.spinee.2004.05.255
Pálfi G, Bereczki Z, Ortner DJ, Dutour O. 2012. Juvenile cases of skeletal tuberculosis from the Terry Anatomical Collection (Smithsonian Institution, Washington, D.C.,USA). Acta Biol Szeged 56:1–12.
Pereira CE, Lynch JC. 2005. Spinal epidural abscess: an analysis of 24 cases. Surg Neurol (Suppl 1) 63:26–29. https://doi.org/10.1016/j.surneu.2004.09.021
Phenice TW. 1969. A newly developed visual method of sexing the os pubis. Am J Phys Anthropol 30:297–302. https://doi.org/10.1002/ajpa.1330300214
Pirpilashviln PM. 1954. On the study of diseases of the bone system on the archaeological materials of the Samtavroi burial ground. Reports of the Academy of Sciences of the Georgian SSR XV (8):551–559.
Pirpilashvili PM. 1956. Traces of some diseases according to paleoanthropological materials. Reports of the Academy of Sciences of the Georgian SSR XVII (4):369–376.
Ratobylsky GV, Khovrin VV, Kamalov YR, Baturin OV, Flerov KE, Mozhokina GN. 2012. Clinical and radiation diagnostics of spinal tuberculosis at the present stage. Diagnostic and interventional radiology 6(1):19–27.
Reinberg SA. 1955. X-ray diagnostics of diseases of bones and joints. Medgiz: Moscow.
Rogers J, Waldron T. 1995. A field guide to joint disease in archaeology. New York: John Wiley & Sons, Inc.
Rokhlin DG. 1965. Diseases of ancient people. Moscow.
Sapico FL, Montgomerie JZ. 1979. Pyogenic vertebral osteomyelitis: report of nine cases and review of the literature. Rev Infect Dis 1:754–776. https://doi.org/10.1093/clinids/1.5.754
Smith EG, Dawson WR. 1924. Egyptian Mummies. George Allen & Unwin Ltd: London.
Talantov VA. 1961. Morphological characteristics of tuberculosis of the spine. Archiv pathol 9:25.
Tesi C, Giuffra V, Fornaciari G, Larentis O, Motto M, Licata M. 2019. A case of erosive polyarthropathy from Medieval northern Italy (12th–13th centuries). Int J Paleopathol 25:20–29. https://doi.org/10.1016/j.ijpp.2019.03.002
Tikhodeev SA, Vishnevsky AA. 2004. Nonspecific osteomyelitis of the spine. Spbmapo: St. Petersburg.
Torres-Gonzalez P, Cervera-Hernandez ME, Martinez-Gamboa A, Garcia-Garcia L, Cruz-Hervert LP, Bobadilla-Del Valle M, Ponce-de Leon A, Sifuentes-Osornio J. 2016. Human tuberculosis caused by Mycobacterium bovis: a retrospective comparison with Mycobacterium tuberculosis in a Mexican tertiary care centre, 2000–2015. BMC Infect Dis 16(1):657. https://doi.org/10.1186/s12879-016-2001-5
Trotter M, Gleser GC. 1958. A reevaluationof estimation of stature based on measurements of stature taken during life and of long bones after death. Am J Phys Anthropol 16:79–123. https://doi.org/10.1002/ajpa.1330160106
Turunc TA, Demiroglu YZ, Uncu H, Colakoglu S, Arslan H. 2007. Comparative analysis of tuberculous, brucellar and pyogenic spontaneous spondylodiscitis patients. J Infect 55:158–163. https://doi.org/10.1016/j.jinf.2007.04.002
Vasilyev SV, Bulgin DV, Simavonyan KV, Borutskaya SB, Emelyanchik OA, Oganesyan AO, Kartashov SI, Chichaev IA. 2022. Experience in the study of paleopathology of the spine using computed tomography and radiography. Bulletin of Archaeology, Anthropology and Ethnography 3(58):136–147.
Yang X, Zhang Q, Guo X. 2014. Value of magnetic resonance imaging in brucellar spondylodiscitis. Radiol Med 119:928–933. https://doi.org/10.1007/s11547-014-0416-x
Yoo S, Wu QJ, Godfrey D, Yan H, Ren L, Das S, Lee WR, Yin F-F. 2009. Clinical Evaluation of positioning verification using digital tomosynthesis (DTS) based on bony anatomy and soft tissues for prostate imageguided radiation therapy (IGRT). Int J Radiat Oncol Biol Phys 73(1):296–305. https://doi.org/10.1016/j.ijrobp.2008.09.006
Zink AR, Reischl U, Wolf H, Nerlich AG. 2002. Molecular analysis of ancient microbial infections. FEMS Microbiology Letters 213(2):141–147. https://doi.org/10.1111/j.1574-6968.2002.tb11298.x

