Available online at: https://doi.org/10.18778/1898-6773.87.1.02
 https://orcid.org/0009-0009-9982-2189
https://orcid.org/0009-0009-9982-2189
Department of Anthropology, Institute of Biomedical Sciences, University of Physical Education in Krakow, 31-571 Krakow, Ave. Jana Pawla II 78
 https://orcid.org/0000-0001-7830-5726
https://orcid.org/0000-0001-7830-5726
Department of Anthropology, Institute of Biomedical Sciences, University of Physical Education in Krakow, 31-571 Krakow, Ave. Jana Pawla II 78
 https://orcid.org/0000-0001-5006-0798
https://orcid.org/0000-0001-5006-0798
Department of Anthropology, Institute of Biomedical Sciences, University of Physical Education in Krakow, 31-571 Krakow, Ave. Jana Pawla II 78
Faculty of Health Sciences, University of Applied Sciences in Tarnow, St. Mickiewicza 8
 https://orcid.org/0000-0003-1548-6536
https://orcid.org/0000-0003-1548-6536
Department of Anthropology, Institute of Biomedical Sciences, University of Physical Education in Krakow, 31-571 Krakow, Ave. Jana Pawla II 78
ABSTRACT: Despite contradictory observations, it has been postulated that early age of adrenarche predisposes to an increased risk of cardiometabolic complications in further ontogeny due to greater body fatness. The aim of this study was to test the above postulates.
We present the results of research on 67 men aged 50–52 – participants of the Krakow Longitudinal Study conducted in the years 1976–2022 – from two birth cohorts 1970 and 1972. Boys were examined annually, aged 6–18, initially 940 people, at the age of eighteen – 358. They were examined again as adult men in 2004 (age 32–34) – 122 people and again in 2022 (age 50–52 years) 67 men. Based on the pubarcheal age, 50-year-olds were divided into 3 groups: early (11 people), average maturing (44 people) and (12 people), where the following were compared: resting systolic and diastolic blood pressure, basic parameters lipid profile – total cholesterol and its fractions, triglycerides, fasting glucose, body height and weight, waist and hip circumferences, indicators – Body Mass Index (BMI), Waist–hip Ratio (WHR), the thickness of 6 skinfolds and the prevalence of metabolic syndrome.
The results of the analyses showed that:
(1) there is a clear gradation, i.e., the earlier the age of pubarche, the worse the metabolic health of men;
(2) compared to the other groups, the total adiposity in men with early pubarche is slightly higher, with clearly marked abdominal obesity; BMI and WHR showed a contrasting picture.
At this stage of the analyses, it is difficult to clearly judge whether the cause of the increased cardiometabolic risk in the studied men with early pubarche is related to earlier age of adrenarche and the mechanisms and stimuli causing it, or to greater adiposity.
KEY WORDS: early adrenarche, metabolic syndrome, abdominal adiposity, blood pressure, triglyceride.
Two independent processes characterise the period of puberty. The first one is adrenarche, during which there is an increase in the activity of the adrenal glands and the secretion of steroid hormones; the second one – gonadarche – is characterised by an increase in the activity of the gonads. Witchel et al. (2020) state that the physiological mechanisms governing the onset of adrenarche remain unclear.
Adrenarche – chemical changes within the endocrine system, invisible at the macro level, observed in boys on average at the age of 7–8, which precede the visible changes in body morphology, i.e. increased growth rate, appearance of acne, increased activity of sweat glands, development of pubic hair – pubarche by approximately 1.5–2 years. Increasing production of adrenal androgens causes these physical manifestations of adrenarche. Research from recent years shows that the dominant bioactive androgen in children during normal and premature adrenarche is 11-ketotestosterone (Rege et al. 2018). After excluding diseases that increase the concentration of adrenal steroid hormones, e.g., androgen-secreting tumours, Cushing’s syndrome and rare genetic disorders, premature adrenarche (PA) is currently considered as a variant of puberty (Potau et al. 1999; Utriainen et al. 2009) – before the age of 7. with. – and, consequently, premature pubarche (PP) – before the age of 9. According to Rosenfield (2021), the cause of premature adrenarche is still unknown. It is often associated with unfavourable metabolic features in children, such as hyperinsulinism, dyslipidemia, obesity (especially abdominal obesity) and later ovarian hyperandrogenism (Utriainen et al. 2015). Potau et al. (1999) observed that premature pubarche in boys, unlike girls, is not associated with a group of endocrine-metabolic abnormalities. Williams et al. (2015) found that metabolic syndrome was only observed in obese boys and girls, regardless of whether they had PA or not. Therefore, obesity appears to increase metabolic risk in the prepubertal population and not in PA. However, one study on a group of boys and girls conducted by Matzarapi et al. (2022) showed that PA-induced metabolic changes begin in childhood.
Do metabolic changes detected in children with PA persist into adulthood? Despite the existence of contradictory results (Bell et al. 2018), some authors postulated that early age of puberty in children predisposes to an increased risk of cardiometabolic complications in further ontogenesis, which is associated with higher adiposity (Widén et al. 2012; Prentice and Viner 2013). However, the authors of these studies used differing determinants of the pace of puberty, i.e., age at menarche (retrospective – recognising that it may be subject to memory errors), age at the peak of the pubertal spurt, skeletal age and others. In a review article, Rosenfield (2021) stated that the effects of premature adrenarche in adults have not been clearly defined.
Due to the existence of the mentioned above discrepancies in study findings, the aim of the presented study was to determine the following:
Due to the increasing prevalence of disorders in this area of health, the results of this study might be useful in early prevention, including mental health. Since the age of pubarche in boys worldwide has significantly decreased over the last few decades, these problems may affect an increasing number of boys and, later in life, men. For instance, over the period 40 years (1971–2010), in the Krakow population the age at the onset of pubic hair development decreased by more than one year: from 12.99 to 11.80 years (Kryst et al. 2012). Such a rapid acceleration of physical maturation is not accompanied by faster mental, emotional and cognitive development because this requires experience and, as stated by, among others, Dorn et al. (1999), Sontag-Padilla et al. (2012), Barendse et al. (2018), Marakaki et al. (2018), Ellis et al. (2019) and Michielsen et al. (2020), early puberty increases the risk of mental health disorders, such as anxiety, depression, oppositional defiant disorders, as well as antisocial and destructive behaviour. Similar association has been found regarding adrenal hormones, such as androgens and cortisol (Michielsen et al. 2020).
The research material consisted of data from a longitudinal study focused on somatic development and physical fitness of girls and boys, conducted in 1976–2022 by the Department of Anthropology of the Institute of Biomedical Sciences of the University School of Physical Education in Krakow (Krakow’s Continuous Research [KBC 1976–2022]). The first series of annual surveys was performed in 1976–1988 (the age of the participants was 6–18 years), and the second series in the years 1980–1990 – the age of the respondents was 8–19 years. The present study combined data from men from two series examined in 2004 (32–34 years old) and re-examined in 2022 (50–52 years old). The number of examined boys and men are as follows:
The numerical statement shows that the number of men in subsequent studies decreased, which is the expected trend in longitudinal studies. The consent of the Bioethics Committee at the Regional Medical Chamber in Krakow was obtained for the examination in 2022 (consent no. 65/KBL/OIL of April 11, 2022). All procedures contributing to the study complied with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
There was no morphological selection of men who participated in research conducted in 2022. The mean body height of 122 men studied in 2004 was 178.1 cm, and 177.9 cm for 67 study participants. The examined men were born in Krakow at the same time (1970 and 1972), grew up in a similar socio-economic reality and during childhood (developmental period) lived in the same district of Krakow (Nowa Huta) consisting of large blocks of flats, near a huge metallurgical and coking complex (the Vladimir Lenin Steelworks in Nowa Huta); they grew up and matured in the 70s and 80s of the 20th century. The first half of the 70s in Poland is often referred to as the “golden Gierek era”, a period of temporary prosperity of the Polish society, during which a marked rise in the standard of life of the citizens was observed and a system of social care, such as free schooling system, free national healthcare system, hygiene-medical and dental care of children and youth in Polish schools, was developed. The modernization and industrialization of the country progressed. Since the mid-50s of the 20th century a number of industrial giants operated in Krakow and its immediate vicinity. During this period factory plants gradually increased their production, resulting in the discharge of many harmful substances into the environment, polluting the soil, water and air. An increase of morbidity rate among both adults and children became a problem which eventually forced the authorities to close down the department of electrolysis in the Aluminum Plant in Skawina (operated on the basis of obsolete Soviet technologies) in1981.
During annual examinations of children, anthropometric measurements were taken, such as body height (b-v distance), length of lower limbs (b-sy), body weight, waist and hip circumferences and thickness of skin-fat folds. In addition, in boys pubic hair development was assessed using the Tanner scale, which covers the period from pre-pubertal (stage I) to adult (stage V) – photos and drawings. The assessment was carried out by designated staff members. In addition to analogous anthropometric measurements, during the study of adult participants of this research (2022) blood pressure was measured (upper arm blood pressure monitor Omron M3 COMFORT). Body weight was measured using a Tanita Body Composition Analyser, type BC-418 MA.
Based on the age of pubarche, the participants were divided into three groups:
In each of the three groups the following morphological characteristics were analysed: body height and weight, length of lower limbs (B-sy distance); waist and hip circumferences; thickness of 6 skinfolds, i.e., above the biceps and triceps, subscapular, abdominal, suprailiac and calf (measured with Harpenden skinfold calliper) and biomedical parameters: resting systolic (SBP) and diastolic (DBP) blood pressure, lipid profile: total cholesterol and its fractions (HDL, non-HDL, LDL), triglycerides and glucose concentration (blood taken on an empty stomach). The analyses were performed by the SYNEVO laboratory in Krakow.
To show the overall length proportions, the lower limb length index (relative legs length) was calculated: (B-sy/body height) x100. Body mass and the type of fat distribution around the waist and hips were estimated in accordance with WHO recommendations, based on body mass index (BMI) and the ratio of waist circumference to hip circumference (WHR).
To illustrate the level and distribution of subcutaneous fat, the following body fat indexes were constructed:
The distribution of subcutaneous fat, the relationship between peripheral fat (limbs) and central fat (trunk), was presented in the form of two indicators:
The occurrence of metabolic syndrome (MS) was verified based on the presence of at least 3 of the following five factors, without indicating the dominant pathogenetic factor; the criteria were chosen following Kramkowska and Czyżewska (2014) and Dobrowolski et al. (2022):
Pearson’s chi-square tests and ANOVA were used to analyze the differences between the singled-out groups of men. The calculations were conducted using the STATISTICA software, version 13 package. The MedCalc statistical software package for biomedical research (version 22.017) was used to analyze the odds ratio (OR) (MedCalc Software Ltd.).
To present a significant amount of information about various features of the body structure of the participants in one place, the results of the analysis of differences between groups were presented in the form of standardised profiles (Fig. 1 and Fig. 2). The mean values of individual characteristics of men with early and average age at pubarche (X) were standardised for the mean (µ) and standard deviation (SD) of the late pubarche age group (“Z-Score” standardization). Z-score was calculated according to the formula:
Z = X – µ / SD,
where:
Z – is the Z-score (standard score), i.e., the number of standard deviations by which a given value of, for example, the body height of individuals in the early or average pubarche group differs from the average body height of individuals with late pubarche,
X – non-standardized variable – score, i.e., the body height values of males from early and late pubarche groups.
If the Z value is positive, it means that the score is above the average value for the reference group, while a negative Z value indicates that the score is below the average value.
Men entering pubarche late were characterised by the best (“healthiest”) biomedical profile in terms of lipid profile components and blood pressure. Therefore, they were used as a reference group. Information about the level of education of the men was also provided. The descriptive characteristics of the analysed features were presented in summary tables.
The average age of initiation of pubic hair development among 50–52-year-old men from Krakow’s Continuous Research (KBC 1976–2022) was calculated using the probit analysis method; median Me = 12.51 years, SE = 0.10, which is almost identical to the result estimated for the entire study population during childhood N = 485, where Me = 12.49 years, SE = 0.05 (Bocheńska and Chrzanowska 1993). Table 1 shows the distribution of the rate of pubarche in subsequent ages in the studied adult men.
The level of education of the examined men is presented in Table 2. In all pubarche age groups, most people had higher education, while vocational education was the least prevalent. Differences in the level of education between age at pubarche groups were relatively small and statistically insignificant (chi2 = 1.082, p = 0.897). The extreme maturation groups – early and late pubarche – were characterised by an almost identical distribution of individual education categories: over 60% with higher education (63.6% and 66.7%, respectively), 27.3% and 27.5.0% with secondary education while 9.1% and 8.3% with vocational education. Among people exhibiting an average pubarche age, there was about 10% fewer in the tertiary education category, but about 10% more had a secondary education. The number of individuals with vocational education was the most similar in each maturation category (Tab. 2).
| Age* (years) | Total number | Post pubarche | ||
| n | cumulation | % | ||
| 9.5 | 67 | 0 | 0 | 0.00 |
| 10.5 | 67 | 3 | 3 | 4.48 |
| 11.5 | 67 | 8 | 11 | 16.42 |
| 12.5 | 67 | 27 | 38 | 56.72 |
| 13.5 | 67 | 17 | 55 | 82.09 |
| 14.5 | 67 | 7 | 62 | 92.54 |
| 15.5 | 67 | 3 | 65 | 97.01 |
| 16.5 | 67 | 2 | 67 | 100.00 |
* middle of the age range
| Pubarcheal age | Level of education * | ||||
| higher | secondary | vocational | Total | ||
| Early | n | 7.0 | 3.0 | 1.0 | 11.0 |
| % | 63.6 | 27.3 | 9.1 | 100.0 | |
| Average | n | 24.0 | 17.0 | 3.0 | 44.0 |
| % | 54.6 | 38.6 | 6.8 | 100.0 | |
| Late | n | 8.0 | 3.0 | 1.0 | 12.0 |
| % | 66.7 | 25.0 | 8.3 | 100.0 | |
* The differences between groups not statistically significant, chi2 = 1.082, P = 0.897
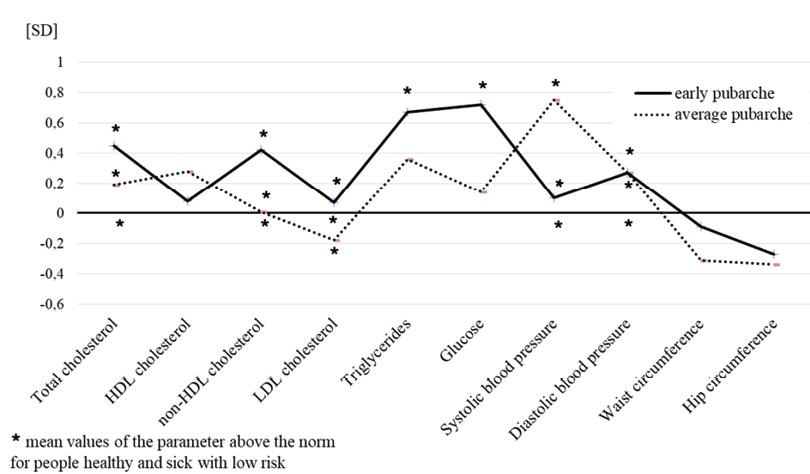
Figure 1 shows, in the form of normalised profiles, the differences between three groups of men exhibiting different pubarcheal ages in terms of lipid profile components, systolic and diastolic blood pressure, waist and hip circumferences.

Fig. 1. The differences in terms of lipid profile components, systolic and diastolic blood pressure, and waist and hip circumferences between men aged 50–52 from the early and average pubarche groups in relations to the late pubarche category – normalised profiles. * Biomedical parameters, whose reference values (medical norms for healthy subjects and low cardiovascular risk group) are exceeded at the level of the average value by the groups with different pubarcheal age, are marked with an asterisk. (KBC 1976–2022)
| Biomedical and physique parameters | Age at pubarche | Differences | ||
| early | average | late | P # | |
| Total cholesterol (mmol/l) | 5.58 (1.24) | 5.33 (1.18) | 5.15 (0.96) | 0.680 |
| 5.40 (4.20; 6.30) | 5.20 (4.70; 6.00) | 5.00 (4.45; 5.95) | ||
| HDL cholesterol (mmol/l) | 1.35 (0.25) | 1.42 (0.26) | 1.32 (0.36) | 0.503 |
| 1.40 (1.22; 1.47) | 1.40 (1.30; 1.66) | 1.20 (1.01; 1.48) | ||
| non-HDL cholesterol (mg/dl) | 163.65 (50.87) | 151.30 (45.96) | 147.83 (37.89 | 0.679 |
| 157.0 (126.0; 190.0) | 147.0 (124.0; 176.0) | 140.0 (117.0; 183.0) | ||
| LDL cholesterol (mmol/l) | 3.29 (0.95) | 3.14 (0.92) | 3.11 (0.99) | 0.883 |
| 3.40 (2.30; 4.00) | 3.10 (2.40; 3.70) | 2.80 (2.40; 4.10) | ||
| Triglycerides (mmol/l) | 1.81 (1.33) | 1.59 (0.87) | 1.34 (0.70) | 0.505 |
| 1.30 (1.10; 2.00) | 1.40 (1.00; 1.90) | 1.20 (0.80; 1.50) | ||
| Glucose (mmol/l) | 5.76 (1.05) | 5.47 (0.57) | 5.40 (0.50) | 0.407 |
| 5.50 (5.20; 9.00) | 5.40 (5.10; 5.70) | 5.40 (5.00; 5.80) | ||
| Systolic blood pressure (mmHg) | 139.18 (14.10) | 147.70 (18.00) | 137.83 (13.10) | 0.477 |
| 134.0 (129.0; 152.0) | 148.5 (137.5; 158.0) | 135.5 (127.5; 141.5) | ||
| Diastolic blood pressure (mmHg) | 92.00 (7.60) | 92.00 (12.00) | 89.70 (8.40) | 0.615 |
| 93.0 (88.0; 99.0) | 91.0 (85.0; 97.0) | 87.5 (83.0; 97.5) | ||
| Waist circumference (cm) | 97.77 (10.08) | 94.76 (11.10) | 98.96 (13.50) | 0.114 |
| 98.5 (91.5; 104.0) | 94.2 (89.2; 101.0) | 96.7 (90.0; 106.5) | ||
| Hip circumference (cm) | 101.50 (7.16) | 100.95 (7.94) | 103.50 (7.38) | 0.803 |
| 103.0 (94.0; 109.0) | 100.7 (97.7; 104.2) | 104.0 (97.5; 108.2) | ||
# ANOVA test (analysis of variance)
In terms of lipid profile components at the level of average values (Fig. 1, Tab. 3), men from all maturation groups exceeded the norms for total cholesterol concentration, HDL and LDL. Triglyceride and glucose levels were additionally exceeded only by early maturing men. Thus, the group with early pubarche had the worst metabolic profile, deviating from the group with late pubarche (i.e., exhibiting the best metabolic condition) the most, within the range of +0.10 to +0.72 SD. The group with average pubarche age deviated slightly less, from -0.08 to +0.36 SD. Only the mean content of HDL was normal in all groups, and the differences between the categories ranged from +0.08 to +0.38 SD. Mean arterial blood pressure was elevated in both SBP (⩾130 mmHg) and DBP (⩾85 mmHg) in all three groups of men. The group with late pubarche had the lowest SBP and DBP, while slightly higher SBP and DBP values were exhibited by the group with early pubarche. The largest deviation (+0.8 SD) regarding SBP, averaging 147 mmHg, was exhibited by the group of average maturing age. Compared to late maturing men, men of early and average pubarche exhibited a worse metabolic condition and were characterised by a slightly smaller average waist and hip circumference as well as more clearly marked in the average age at pubarche category (Fig. 1, Tab. 3).
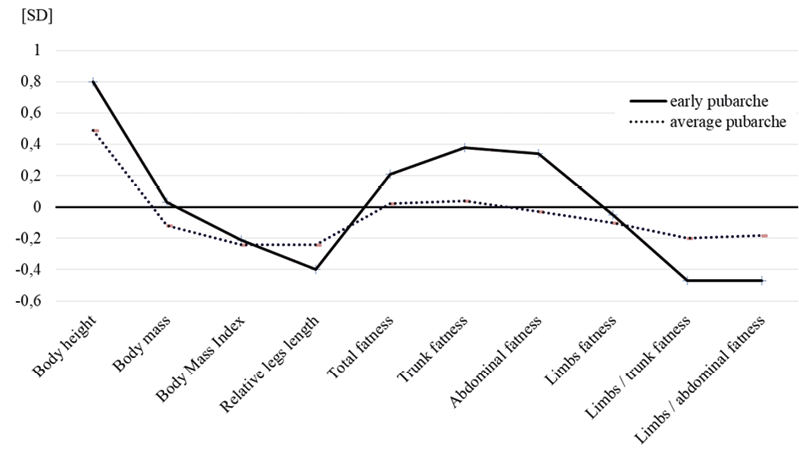
Fig. 2. The differences in selected features of body morphology between men aged 50–52 from the early and average pubarche groups in relations to the late pubarche category – normalised profile. (KBC 1976–2022)
| Physique parameters | Age at pubarche | Differences | ||
| early | average | late | P # | |
| Height (cm) | 179.25 (8,07) | 178.02 (5.66) | 176.02 (4.05) | 0.803 |
| 180.5 (175.4; 186.2) | 177.9 (174.2; 181.7) | 177.6 (173.0; 179.2) | ||
| Weight (kg) | 89.48 (15.68) | 87.59 (15.24) | 89.43 (15.63) | 0.516 |
| 91.9 (74.8; 102.0) | 87.3 (78.6; 93.9) | 86.2 (77.65; 102.2) | ||
| BMI (kg/m2) * | 27.76 (3.58) | 27.59 (4.39) | 28.96 (5.76) | 0.473 |
| 27.9 (24.1; 31.0) | 28.0 (24.4; 30.15) | 27.0 (25.45; 32.0) | ||
| Relative legs length ** (%) | 52.51 (1.60) | 52.65 (1.18) | 52.86 (0.88) | 0.936 |
| 52.4 (51.2; 53.9) | 52.7 (51.9; 53.6) | 52.9 (52.1; 53.7) | ||
| Total fatness (cm) *** | 10.09 (2.17) | 9.44 (3.03) | 9.38 (3.31) | 0.270 |
| 10.1 (7.7; 11.8) | 9.0 (7.5; 11.5) | 9.3 (6.2; 11.9) | ||
| Trunk fatness (cm) | 7.00 (1.42) | 6.28 (1.94) | 6.20 (2.10) | 0.306 |
| 6.9 (5.2; 8.3) | 6.1 (5.5; 8.6) | 6.1 (4.4; 7.9) | ||
| Abdominal fatness (cm) | 4.78 (1.03) | 4.22 (1.33) | 4.26 (1.52) | 0.424 |
| 4.9 (3.5; 5.6) | 4.2 (3.3; 5.2) | 4.4 (2.9; 5.6) | ||
| Limbs fatness (cm) | 2.35 (0.65) | 2.30 (0.85) | 2.40 (0.99) | 0.899 |
| 2.4 (1.7; 2.8) | 2.1 (1.8; 2.8) | 2.2 (1.5; 3.3) | ||
| Limbs/trunk fatness (%) | 33.31 (5.51) | 36.74 (7.87) | 39.32 (12.70) | 0.667 |
| 33.7 (30.1; 37.1) | 35.0 (32.3; 41.0) | 36.8 (29.3; 43.5) | ||
| Limbs/abdominal fatness (%) | 49.10 (8.53) | 54.50 (12.63) | 57.90 (18.9) | 0.792 |
| 50.0 (42.0; 53.9) | 53.2 (46.2; 63.3) | 57.1 (45.1; 61.6) | ||
* Body Mass Index; ** (B-sy/body height) x100; *** individual measures of body fatness are presented in the methods;
# ANOVA test (analysis of variance)
Figure 2 illustrates the differences, in the form of normalised profiles, among selected features of body morphology between men from the early and average pubarche groups in relation to the late pubarche category. The highest average body height was exhibited by the early pubarche group, which was 3 cm higher compared to those with late maturation (+0.8 SD), with the same body weight as the reference group, which translates into a mathematical picture of a slimmer overall body figure in them (BMI was lower by 1.2 kg/m2). However, the average BMI values of both groups were at the upper limit of the overweight category (Fig. 2, Tab. 4). The average lower limb index of -0.40 SD for the early pubarche age indicated that, given their greater average body height, study participants belonging to this category had relatively shorter lower limbs. This is somewhat less marked with regards to the average maturation group. The level and distribution of subcutaneous fat determined by the thickness of the skinfolds also differed. The average total, trunk and abdominal sum of skinfolds are the highest in early maturing men. The deviation of these parameters from the reference group averaged approximately +0.4 SD (difference from 0.5 to 0.8 cm, depending on the skinfold). However, the adiposity of the limbs was identical in both groups. The relationship between the adiposity of the limbs and of both the trunk and the abdomen suggested a greater tendency to accumulate fat in the central rather than peripheral deposits in the early maturing group, deviation +0.47 SD, with differences in the values of the indices of approximately 8%. This does not suggest that there was no such tendency in the other groups but, rather, indicates that this tendency was less pronounced. The average pubarche group was more similar to the early maturing group in terms of general body structure but more similar to the late maturing group regarding adiposity (Fig. 2, Tab. 4).
| Biomedical parameters and physique indexes | Age at pubarche | |||
| early | average | late | ||
| Elevated total-cholesterol > 5.0 mmol/l |
n | 8/11 | 28/43 | 6/12 |
| % | 72.7 | 65.1 | 50.0 | |
| Low HDL-cholesterol < 1.0 mmol/l |
n | 2/11 | 2/43 | 2/12 |
| % | 18.2 | 4.6 | 16.7 | |
| Elevated non-HDL cholesterol > 145 mg/dl |
n | 7/11 | 24/43 | 6/12 |
| % | 63.6 | 55.8 | 50.0 | |
| Elevated LDL-cholesterol > 3.0 mmol/l |
n | 7/10 | 31/42 | 7/12 |
| % | 70.0 | 73.8 | 58.3 | |
| Elevated triglyceride > 107 mmol/l |
n | 5/11 | 11/43 | 2/12 |
| % | 45.4 | 32.6 | 16.7 | |
| Elevated glucose level ⩾ 5.6 mmol/l |
n | 5/11 | 19/43 | 4/12 |
| % | 41.7 | 44.2 | 30.0 | |
| High systolic blood pressure ⩾ 130 mm Hg |
n | 8/11 | 38/44 | 8/12 |
| % | 72.7 | 86.4 | 66.7 | |
| High diastolic blood pressure ⩾ 85 mm Hg |
n | 10/11 | 33/44 | 9/12 |
| % | 90.9 | 75.0 | 75.0 | |
| BMI * normal body weight 18.5 – 24.9overweight + obesity ⩾ 25 | n | 3/11 | 12/44 | 3/12 |
| % | 27.3 | 27.3 | 25.0 | |
| n | 8/11 | 32/44 | 9/12 | |
| % | 72.7 | 72.7 | 75.0 | |
| WHR * peripheral fat distribution < 1.0 central fat distribution ⩾ 1.0 | n | 2/11 | 10/44 | 1/12 |
| % | 18.2 | 22.7 | 8.3 | |
| n | 9/11 | 34/44 | 11/12 | |
| % | 81.8 | 77.3 | 91.7 | |
| Metabolic syndrome (MS) ** presence | n | 5/11 | 16/43 | 4/12 |
| % | 45.4 | 37.2 | 33.3 | |
Note: * standards according to Word Health Organization (WHO); BMI – Body Mass Index; WHR – Waist-to--hip ratio; ** verification criteria are described in the methods; presence of ⩾ 3 criteria within the group
Table 5 presents the prevalence of lipid and sugar metabolism abnormalities measured by exceeding the reference norms for a healthy population and low-risk patients, as well as the related unfavourable changes in body structure and arterial blood pressure in groups of men of different pubarcheal age. Compared to men with late pubarche, in the early maturing category, in terms of all biomedical parameters, the number of individuals exceeding the reference norms was higher, i.e., the severity of metabolic abnormalities was greater. Compared to men with late pubarcheal age, the chance of elevated triglyceride level is 2.86 times higher in men with early pubarcheal age (OR = 2.86; 95% CI: 0.35–22.7).
The prevalence of metabolic syndrome in this group was also slightly higher (45.4% vs. 33.3%), which is a logical consequence of the higher prevalence of abnormalities in individual biomedical parameters. Relative to men with late pubarcheal age, the chance of developing MS is 1.67 times higher in men with early pubarcheal age (OR = 1.67; CI: 0.31–9.01, P = 0.553), statistically insignificant. However, unfavourable health changes observed in body structure measured by the BMI showed an excessive body weight and determined by the proportion of waist to hip circumference (WHR ⩾ 1.0) indicating the central (abdominal) fat deposit, occur with a slightly higher frequency in the late maturing group in relation to other groups. For most of the analysed parameters, men of average pubarcheal age exhibited an intermediate prevalence of the analysed abnormalities (Tab. 5).
The aim of the present study was to determine (1) whether early age of adrenarche in boys, calculated as the age of physical manifestation of this phenomenon in the form of pubarche, is associated with a greater risk of cardiometabolic complications in adulthood, (2) whether early age of adrenarche is related to increased adiposity. There are few studies on this topic, especially regarding the male gender. This form of longitudinal research constituting systematic annual observations regarding the same group of study participants at the age from 6 (adrenarche time) to 18 years (the time of adolescence), followed by taking data on the same study participants at 30-year-olds and 50-year-olds, has not been found in the available literature. The discussion of the topic is limited.
Sixty-seven men, mostly with higher education (over 60%), participated in the last stage of the study, and groups with extreme maturation ages, early and late pubarche categories, were characterised by an almost identical distribution of individual education categories (Tab. 2). Suppose we assume that the level of education determines the intellectual level. In that case, it can be roughly said that the intellectual level of the respondents was similarly diverse, which may translate into comparable knowledge about the need for health-promoting behaviours. Such a high percentage of higher education exceeded the average for large cities and, to an even greater extent, the national average.
Regarding metabolic health, the early pubarche group had the greatest burden and the late pubarche group exhibited the best health parameters. At the level of average values, all parameters of the lipid profile, glucose concentration, SBP and DBP had higher values in the early pubarche group compared to the late pubarche category (reference group), deviating from +0.07 SD (LDL) to +0.7 SD for triglycerides and glucose and +0.4 SD for cholesterol and triglycerides (Fig. 1, Tab. 5). Similarly, compared to men with late pubarche, in the group with early maturation, the percentage of men exceeding the reference norms was higher regarding all biomedical parameters (Tab. 5). Thus, early pubarche was accompanied by a greater severity of metabolic abnormalities. The average maturation group was more similar to those in the late pubarche category. Therefore, there was a clear gradation: the earlier the age of pubarche, the worse the metabolic health of men and, consequently, the greater the risk of cardiovascular events (none of the studied men had premature pubarche).
Such a different metabolic picture presented by the separate groups of pubarche in terms of trends, even (or perhaps even more so) in the face of a small sample, allowed us to conclude that there is a relationship between the age of initiation of puberty (pubarche, and therefore the preceding adrenarche) and men’s metabolic health.
The worse metabolic condition of the surveyed early maturing men is logically associated with the highest incidence of metabolic syndrome (MS). Therefore, MS was found to be the most common among them (45.4%), and among late maturing, the prevalence was the lowest (33.3%) (Tab. 5). The MS includes selected biomedical characteristics, i.e. increased triglyceride concentration, decreased HDL concentration, increased blood pressure – systolic and/or diastolic, abnormal fasting glycemia and increased waist circumference signalling abdominal obesity (Kramkowska and Czyżewska 2014). Their co-occurrence increases the risk of developing atherosclerotic cardiovascular diseases and type 2 diabetes (Kramkowska and Czyżewska 2014; Banach et al. 2021; Dobrowolski et al. 2022). Contrary to what could be expected, average absolute waist circumference was not the largest in men with the poorest metabolic health but in the healthiest ones – those with late pubarche. A similar situation concerned hip circumference (Fig. 1, Tab. 3). Therefore, WHR, commonly considered a determinant of fat distribution, showed a slightly higher prevalence of the central (abdominal) type of fat distribution, associated with a higher risk of metabolic disorders, in individuals with late rather than early pubarche (Fig. 2, Tab. 5).
Similarly, the average BMI was lower in men of early and average pubarcheal age compared to late maturing men, who were more corpulent according to this index (Fig. 2, Tab. 4). This results from a subtle mathematical play of body height and weight, where the squared value of body height significantly influences the quotient. In the study sample, early maturing men were, on average, 3 cm taller than those with late pubarche, with almost identical body weight (Tab. 4). According to some authors (Bulum et al. 2015), the BMI does not reflect the distribution of fat tissue, and the use of waist and hip circumference and WHR has its limitations in diagnosing central obesity. The value of waist circumference may be inadequate for exceptionally short or tall people with similar values of this measurement. In the light of our research, these statements seem to be correct. The results of the analysis of the level and distribution of subcutaneous fat based on the measurement of skinfold thickness seem to be in contrast to the assessment employing BMI and WHR (Fig. 2, Tab. 4). The average absolute value of the total sum of six skinfolds, the trunk and abdomen, were the highest in early maturing men (difference in skinfold thickness from 0.5–0.8 cm). However, the adiposity of the limbs was identical in both groups. The ratios of adiposity of the limbs to both the trunk and the abdomen showed a greater tendency to accumulate fat in the central rather than peripheral deposit in the group of early maturing men compared to the other categories (Fig. 2, Tab. 4).
In general, the results of our study show that: (1) adults that experienced early pubarcheal age have an increased risk of metabolic abnormalities and, consequently, a higher risk of cardiovascular events; (2) compared to individuals with a later pubarcheal age, subcutaneous fatness estimated from the skinfold thickness is slightly higher, with clearly marked abdominal obesity; (3) the results of body fat estimates in the form of BMI and WHR do not fully correspond to the results of skinfold-based measurements, which makes the interpretation of the findings more difficult.
The results of our study do not allow us to conclusively state whether the cause of the increased risk of cardiometabolic disorders in the examined men with early pubarcheal age is the mere fact of earlier adrenarcheal age and the mechanisms and stimuli causing it, or whether it is necessary for the early pubarche to coexist with the high fatness.
There are few reports on PA in boys. Data from the available literature regarding both sexes are contradictory. The international dialogue on the topic of the searly age of adrenarche as a potential factor, the source of various disorders in the functioning of the child’s body at different stages of ontogenesis, is briefly presented in the introduction. There have been many studies investigating the relationship between the advancement of sexual development and metabolic disorders and other dysfunctions, such as mental diseases, although they concerned other later stages of puberty based not on the pubarche’s age but on the timing of the voice changes (mutation), later stages of pubic hair development, and the pace of puberty measured by the time of the onset of pubertal growth spurt. Various methods of assessing adiposity are used, most often based on BMI and WHR. The later phases of puberty are under the control of other areas of hormonal regulation – the HPG axis (hypothalamus-pituitary-gonadal), while pubarche is regulated by the HPA axis hypothalamus-pituitary-adrenal). Additionally, the results often depend on the research methods used in the study. According to some authors (Havelock et al. 2004; Belgorosky et al. 2008; Styne 2011) the time of the onset of adrenarche has no significant effect on the age of onset of gonadarche. Much evidence supports the existence of separate mechanisms controlling these two pubertal events.
Hormonal products of the adrenal cortex (HPA axis), that manage adrenarche participate not only in the child’s sexual development but also in the regulation of metabolism, blood pressure, and, what is important, in managing the body under stress of chemical, physical and emotional nature (Carroll et al. 2011; Javorsky et al. 2011). The acceleration in the timing of increasing the body’s androgen production, apart from the early appearance of pubic hair, often causes metabolic disorders similar to those that can be present in adults. This seems understandable due to the range of body functions under the adrenal glands’ control, which may be disturbed by some individual or parallel stimulus/stimuli or exist in a cause-and-effect relationship.
Emotional stressors may be one type of such stimuli. Analysis of pubic hair development in a sample of 1146 school-age boys from Krakow (cross-sectional study from 2010) showed that, compared to their peers from complete families, boys growing up in single-parent families have a markedly accelerated development. Their pubarcheal age begins, on average, over a year earlier (10.70 years vs. 11.95 years) (Kowal et al. 2011a). Differences between them were also marked at the stage of intrauterine development. As newborns, they were characterised by a slightly smaller size compared to their peers from full families, with a slightly shorter gestation time (there were no cases with a weight below 2,500 g). However, their mothers and fathers were slightly taller and slimmer. The differences discussed were not large (-0.2 to -0.3 SD) and were statistically insignificant, except for weight gain during pregnancy – single mothers gained significantly more weight. The incidence of complications of pregnancy and delivery was similar. An in-depth analysis of pregnancy-related stress, assessed subjectively by mothers, showed a statistically significantly higher magnitude and frequency among women with single-parent family status, as well as a worse assessment of both family and material situation. In addition, single mothers were twice as likely to admit to smoking cigarettes during pregnancy. Stress and smoking during pregnancy have been shown to exert considerable adverse effects on offspring (Kowal et al. 2011b). It should be noted that not all married women are happy mothers. Despite existing stressful family conflicts, a significant number of mothers do not choose to separate or divorce.
Many studies have linked an unfavourable prenatal environment to the development of cardiometabolic disorders and neuroendocrine dysfunctions, as well as an increased risk of mental illness in later life (Van den Bergh et al. 2020; Eberle et al. 2021). Experiments in animal models have shown that prenatal exposure to stress or excess glucocorticoids can disturb the physiology of the offspring, resulting in reduced birth weight and consequently an increased likelihood of cardiovascular dysfunction, glucose homeostasis, HPA axis activity and anxiety-related behaviour in adulthood (‘developmental programming’ theory). Glucocorticoids are key mediators of stress responses mediating developmental programming (Cottrell and Seckl 2009).
Stress enhances the production of the CRH hormone (corticoliberin) in the hypothalamus, which stimulates the pituitary gland to produce adrenocorticotropic hormone (ACTH). ACTH subsequently stimulates the production and secretion of glucocorticosteroids, mineralocorticosteroids and androgens by the adrenal cortex in a related manner; there is no separate pituitary hormone inducing adrenal androgen production. Instead, inhibition of hormone secretion is mediated by negative feedback via the action of glucocorticosteroids on ACTH secretion (Javorsky et al. 2011). Thus, one could hypothetically assume that in boys entering school age and growing up in unfavourable family conditions exacerbating stressful situations, the HPA axis causes, in addition to an increase in cortisol, an increase in the production of androgens (adrenarche) that regulate pubic hair development (pubarche).
Children growing up amid strained family relationships and facing family breakdown are at risk of serious emotional and behavioural disorders, including depressive disorders, learning difficulties, and problems in relationships with peers as well as parents (Schor 2003). In a review article, Pietrzak (2020) showed that obesity in children co-occurs with their poorer emotional functioning. Several authors indicated that it is obesity which is the cause of this situation. Obese children are often discriminated against in their peer environment and experience a lot of psychological distress, which can consequently lead to serious emotional disorders requiring psychotherapeutic intervention. Negative attitudes (verbal and physical violence) towards overweight children start as early as pre-school age and increase among primary school children. Children experience negativity more acutely than adults, which increases their feelings of hopelessness and poor self-esteem (Pietrzak, 2020). In a review of articles, Godina-Flores and co-authors (2023), demonstrated that Mexican children and adolescents who are overweight or obese, are more likely to be depressed and report more depressive symptoms than normal-weight participants (Godina-Flores et al. 2023). Similar effects were observed in adults. A study by Rosmond et al (1998) on a group of 51-year-old men showed that abdominal obesity (and other markers of the metabolic syndrome) is associated with dysregulation of the HPA axis, which follows prolonged over-activation of the axis under factors such as environmental stress. Further analysis by Rosmond et al (1999) revealed that individuals with abdominal obesity show signs of HPA axis dysregulation following chronic, submissive stress, particularly evident in men with impulsive and anxious personality disorders. Men’s perceived environmental stress depends on personality traits. Ortega-Montiel and co-authors (2015) found that self-reported chronic stress was an independent risk factor for obesity in Mexican men. Olszanecka-Glinianowicz (2008) concludes that despite shared pathophysiological pathways (abnormal HPA activation) and similar extrinsic factors influencing the development of obesity and depression, the question which of these diseases is the cause and which is the effect cannot be clearly answered. Still, their co-occurrence may lead to increased mortality, as both obesity and depression are independent risk factors for cardiovascular disease (Olszanecka-Glinianowicz 2008).
The results of our study may point to a similar kind of dilemma. On the one hand, psychological problems caused by obesity (lowered self-esteem, lack of acceptance of one’s appearance and social isolation) can cause increased level of stress, lowered mood, and the occurrence of depression. On the other hand, chronic stress, lowered mood, and the occurrence of depression leading to changes in eating behavior (comforting with food and compulsive eating) may be the cause of the development of obesity (Olszanecka-Glinianowicz 2008). Obesity and depression are different, visible markers of the presence of a stress state in the body (disruption of homeostasis). Analogous conditions and neuroendocrine mechanisms could explain the observed phenomenon of accompanying early pubarche in men by the effect of increased tendency to abdominal obesity. The association between the two variables was found to be statistically insignificant, which may indicate that no such relationship exists. Psychosocial stress can result in accelerated adrenarche and subsequent early pubarche. Stress can also result in the appearance of increased abdominal obesity. Early pubarche and abdominal obesity are different, visible, external markers of the presence of a stress state in the human body. A direct, “statistically visible”, cause-and-effect relationship between the two may not exist, but underlying both phenomena is stress, a disturbance in the activity of the HPA axis. The human body may react differently to prolonged stress with increased appetite or decreased cravings, or the two may alternate during life. As a result, some people will show a tendency to gain weight and others will lose weight.
We do not have more detailed information about the family, psychosocial environment during childhood – the peri-adrenarcheal period of the long-term group of 50-year-olds we studied. Considering the above-cited results of our own research on the Krakow population and world studies, the idea arises that in the early pubarche group more men may have grown up in a disadvantaged psychosocial situation than in the other pubertal groups.
Disturbances in the functioning of the HPA axis, and the subsequent growth and maturation of children, can also occur under the influence of factors of a chemical nature – unfavourable products of industrial activity. Editorial framework does not allow to present here another thread and a hypothetical, no less important disruptor. This will constitute the subject of the next paper, in the context of changes with age of selected body characteristics, including fatness, during the period of 8 to 17 years – the beginning and end of sexual maturation. Were the manifestations of abdominal obesity tendencies in boys already present during the developmental period?
Citing Kramkowska and Czyżewska (2014), metabolic syndrome is the main factor in the development of cardiovascular diseases, which are one of the leading causes of death in Western societies, so it is not surprising that this problem attracts the attention of the global medical community. Nevertheless, so far, the pathogenesis of this syndrome has not been fully understood.
Small sample size and, therefore, the low number of individuals in the extreme maturation groups limited the possibility of conducting statistical analyses and obtaining reliable estimates. The applied Pearson’s chi-square tests and ANOVA showed the lack of statistical significance of differences between groups, even in the case of apparent differences in the mean values of analysed features, but this is the property of statistical tests due to the size of the groups; Therefore, the term “tendencies” was used. This is a limitation of this study.
It was investigated whether the group of 67 men was representative. The average height and weight, BMI, pubarche age and distributions of these characteristics in the group of adult males studied as 17-year-olds were compared to analogous characteristics in the entire group of long-term study boys at 17 years of age. No statistically significant differences were found between the groups.
Nevertheless, such research has great value, is highly time-consuming, takes decades and is labour-intensive and expensive. The studied group has the same, i.e. “closed” gene pool throughout the observation period. During the research, adaptive changes in the phenotype resulting from the interaction of genes with the environment, governed by epigenetic mechanisms that control gene activity, are observed (not the result of gene pool changes, as can occur in cross-sectional studies).
Another advantage is that the group conducting the research consisted mainly of the same people, which is essential, for example, in assessing the development of pubic hair, which is, to some extent, a subjective assessment.
The revealed gradation, the earlier the age of pubarche, the worse the metabolic health of men, takes us back in time and shows us the source of the abnormalities – adrenarche – as a stage in ontogeny; the earlier a child reaches this stage, the worse the health prognosis. However, early adrenarche is not the instrumental cause of the described difficulties; it is a manifestation, a result of the factors causing it; all we need do is discover them. It appears that the stage of adrenarche is a critical, open “developmental window” for cardiometabolic risk for the male sex. Further research is needed.
Acknowledgement
The study was financed within the program of the Ministry of Science and Higher Education in Poland as the ‘Regional Initiative of Excellence’ in the years 2019–2022 (Project No. 022/RID/2018/19) in the amount of 11 919 908 PLN (internal number at University: 35/PB/RID/2022). We would like to thank all research participants for their time and effort in participating in nearly 40 years of research. The authors confirm that neither the manuscript nor any parts of its content are currently under consideration or published in another journal. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Authors’ contribution
BAS was the originator of this study, performer statistical analysis, interpreted the results, drafted the manuscript and wrote the papier; AW performer statistical analysis and interpreted the results; RŻ was the originator of research and manager, and proofreader; MK participated in collecting the bibliography. All authors participated in data collection, confirmed the correctness of the interpretation of the analysis results, revised the manuscript and approved the final version.
Banach M, Burchardt P, Chlebus K, Dobrowolski P, Dudek D, Dyrbuś K, et al. 2021. Wytyczne PTL/KLRwP/PTK/PTDL/PTD/PTNT diagnostyki i leczenia zaburzeń lipidowych w Polsce 2021. Nadciśnienie Tętnicze w Praktyce 7(3):113–222. https://www.nadcisnienietetnicze.pl/sites/scm/files/2022-01/Wytyczne%20PTLKLRWPPTKPTDLPTDPTNT%20diagnostyki%20i%20leczenia%20zaburze%C5%84%20lipidowych%20w%20Polsce%202021.pdf [Accessed 21 October 2023].
Barendse MEA, Simmons JG, Byrne ML, Patton G, Mundy L, Olsson CA, et al. 2018. Associations between adrenarcheal hormones, amygdala functional connectivity and anxiety symptoms in children. Psychoneuroendocrinology 97:156–63. https://doi.org/10.1016/j.psyneuen.2018.07.020
Bell JA, Carslake D, Wade KH, Richmond RC, Langdon RJ, Vincent EE, et al. 2018. Influence of puberty timing on adiposity and cardiometabolic traits: A Mendelian randomisation study. PLoS Med 15(8):e1002641. https://doi.org/10.1371/journal.pmed.1002641
Belgorosky A, Baquedano MS, Guercio G, Rivarola MA. 2008. Adrenarche: postnatal adrenal zonation and hormonal and metabolic regulation. Horm Res 70(5):257–67. https://doi.org/10.1159/000157871
Bocheńska Z, Chrzanowska M, editors. 1993. Rozwój somatyczny, fizjologiczny i psychiczny dzieci i młodzieży o różnym poziomie sprawności fizycznej w świetle badań długofalowych. Wydawnictwo Monograficzne Nr 52 Akademii Wychowania Fizycznego w Krakowie, Kraków. 178.
Bulum T, Blaslov K, Duvnjak L. 2016. The use of anthropometric measurements of obesity in prediction of microvascular complications in obese type 2 diabetic patients. Acta Clin Croat 55(2):217–23. https://doi.org/10.20471/acc.2016.55.02.06
Carroll TB, Aron DC, Findling JW, Tyrrell JB. 2011. Glucocorticoids and adrenal androgens. In: Gardner DG, Shoback D, editors. Greenspan’s Basic & Clinical Endocrinology, 9th Edition. McGraw Hill. 289–292.
Cottrell EC, Seckl JR. 2009. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci 7;3:19. https://doi.org/10.3389/neuro.08.019.2009
Dobrowolski P, Prejbisz A, Kuryłowicz A, Baska A, Burchardt P, Chlebus K, et al. 2022. Zespół metaboliczny – nowa definicja i postępowanie w praktyce. Stanowisko PTNT, PTLO, PTL, PTH, PTMR, PTMSŻ, Sekcji Prewencji i Epidemiologii PTK, „Klubu 30” PTK oraz Sekcji Chirurgii Metabolicznej i Bariatrycznej TChP. Lekarz POZ 3:147–68. https://www.nadcisnienietetnicze.pl/sites/scm/files/2022-07/Zespol_metaboliczny_stanowisko.pdf [Accessed 21 October 2023].
Dorn LD, Hitt SF, Rotenstein D. Biopsychological and cognitive differences in children with premature vs. on-time adrenarche. Arch Pediatr Adolesc Med. 1999;153(2):137–46. https://doi.org/10.1001/archpedi.153.2.137
Eberle C, Fasig T, Brüseke F, Stichling S. 2021. Impact of maternal prenatal stress by glucocorticoids on metabolic and cardiovascular outcomes in their offspring: A systematic scoping review. PLoS One 22;16(1):e0245386. https://doi.org/10.1371/journal.pone.0245386
Ellis R, Fernandes A, Simmons JG, Mundy L, Patton G, Allen NB, Whittle S. Relationships between adrenarcheal hormones, hippocampal volumes and depressive symptoms in children. Psychoneuroendocrinology. 2019;104:55–63. https://doi.org/10.1016/j.psyneuen.2019.02.016
Godina-Flores NL, Gutierrez-Gómez YY, García-Botello M, López-Cruz L, Moreno-García CF, Aceves-Martins M. 2023. Obesity and its association with mental health among Mexican children and adolescents: a systematic review. Nutr Rev 10;81(6):658–669. https://doi.org/10.1093/nutrit/nuac083
Havelock JC, Auchus RJ, Rainey WE. 2004.The rise in adrenal androgen biosynthesis: adrenarche. Semin Reprod Med 22(4):337–47. https://doi.org/10.1055/s-2004-861550
Javorsky BR, Aron DC, Findling JW, Tyrrell JB. 2011. Hypothalamus and Pituitary gland. In: Gardner DG, Shoback D, editors. Greenspan’s Basic & Clinical Endocrinology, 9th Edition. McGraw Hill. 76–78.
Kowal M, Cichocka BA, Woronkowicz A, Pilecki MW, Sobiecki J, Kryst Ł. 2011. Międzypokoleniowe zmiany w budowie ciała i akceleracja pokwitania u dzieci i młodzieży w wieku 7–15 lat z populacji wielkomiejskiej w świetle uwarunkowań psychosocjalnych [Intergenerational changes in body structure and acceleration of puberty in children and adolescents aged 7–15 from the metropolitan population in the light of psychosocial conditions.] red. Kowal M, Cichocka BA. Monografie Nr 5 Akademii Wychowania Fizycznego w Krakowie, Krakow. Kowal a – page 78; Kowal b – page 72. https://www.awf.krakow.pl/pdf/miedzypokoleniowe_zmiany_w_budowie_ciala.pdf [Accessed 7 January 2024].
Kramkowska M, Czyżewska K. 2014. Zespół metaboliczny — historia, definicje, kontrowersje. Forum Zaburzeń Metabolicznych 5(1):6–15. https://journals.viamedica.pl/forum_zaburzen_metabolicznych/article/view/38762 pdf [Accessed 21 October 2023].
Kryst Ł, Kowal M, Woronkowicz A, Sobiecki J, Cichocka BA. 2012. Secular changes in height, body weight, body mass index and pubertal development in male children and adolescents in Krakow, Poland. J Biosoc Sci 44(4):495–507. https://doi.org/10.1017/S0021932011000721
Marakaki C, Pervanidou P, Papassotiriou I, Mastorakos G, Hochberg Z, Chrousos G, et al. 2018. Increased symptoms of anxiety and depression in prepubertal girls, but not boys, with premature adrenarche: associations with serum DHEAS and daily salivary cortisol concentrations. Stress 21(6):564–68. https://doi.org/10.1080/10253890.2018.1484446
Matzarapi K, Giannakopoulos A, Chasapi SA, Kritikou D, Efthymiadou A, Chrysis D, et al. 2022. NMR-based metabolic profiling of children with premature adrenarche. Metabolomics 14;18(10):78. https://doi.org/10.1007/s11306-022-01941-4
MedCalc Software Ltd. Odds ratio calculator. https://www.medcalc.org/calc/odds_ratio.php (Version 22.017) [Accessed 3 January 2024].
Michielsen PJS, Roza SJ, van Marle HJC. 2020. Endocrine markers of puberty timing and antisocial behaviour in girls and boys. Crim Behav Ment Health 30(2–3):117–131. https://doi.org/10.1002/cbm.2149
Olszanecka-Glinianowicz M. 2008. Depresja – przyczyna czy skutek otyłości? [Depression – cause or result of obesity?] Endokrynol Otyłość 4(2):78–85. https://journals.viamedica.pl/eoizpm/article/viewFile/26040/20850 [Accessed 3 January 2024]
Ortega-Montiel J, Posadas-Romero C, Ocampo-Arcos W, Medina-Urrutia A, Cardoso-Saldaña G, Jorge-Galarza E, et al. 2015. Self-perceived stress is associated with adiposity and atherosclerosis. The GEA Study. BMC Public Health 14;15:780. https://doi.org/10.1186/s12889-015-2112-8
Pietrzak A. 2020. Otyłość dziecięca w perspektywie psychospołecznej. Rola edukacji żywieniowej, „Edukacja Elementarna w Teorii i Praktyce” 15;4(58):23–37. https://doi.org/10.35765/eetp.2020.1558.02
Potau N, Ibáñez L, Riqué S, Sanchez-Ufarte C, de Zegher F. 1999. Pronounced adrenarche and precocious pubarche in boys. Horm Res 51(5):238–41. https://doi.org/10.1159/000023377
Prentice P, Viner RM. 2013. Pubertal timing and adult obesity and cardiometabolic risk in women and men: a systematic review and meta-analysis. Int J Obes (Lond) 37(8):1036–43. https://doi.org/10.1038/ijo.2012.177
Rege J, Turcu AF, Kasa-Vubu JZ, Lerario AM, Auchus GC, Auchus RJ, et al. 2018. 11-Ketotestosterone Is the Dominant Circulating Bioactive Androgen During Normal and Premature Adrenarche. J Clin Endocrinol Metab 103(12):4589–98. https://doi.org/10.1210/jc.2018-00736
Rosenfield RL. 2021. Normal and Premature Adrenarche. Endocr Rev 42(6):783–814. https://doi.org/10.1210/endrev/bnab009
Rosmond R, Dallman MF, Björntorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. 1998. J Clin Endocrinol Metab 83(6):1853–9. https://doi.org/10.1210/jcem.83.6.4843
Rosmond R, Eriksson E, Björntorp P. 1999. Personality disorders in relation to anthropometric, endocrine and metabolic factors. J Endocrinol Invest 22(4):279–88. https://doi.org/10.1007/BF03343557
Schor EL. 2003. Family pediatrics: report of the Task Force on the Family. American Academy of Pediatrics Task Force on the Family. Pediatrics 111(6 Pt 2):1541–71.
Sontag-Padilla LM, Dorn LD, Tissot A, Susman EJ, Beers SR, Rose SR. 2012. Executive functioning, cortisol reactivity, and symptoms of psychopathology in girls with premature adrenarche. Dev Psychopathol 24(1):211–23. https://doi.org/10.1017/S0954579411000782
Styne D. 2011. Puberty. In: Gardner DG, Shoback D, editors. Greenspan’s Basic & Clinical Endocrinology, 9th Edition. McGraw Hill. 535.
Utriainen P, Voutilainen R, Jääskeläinen J. 2009. Continuum of phenotypes and sympathoadrenal function in premature adrenarche. Eur J Endocrinol 160(4):657–65. https://doi.org/10.1530/EJE-08-0367
Utriainen P, Laakso S, Liimatta J, Jääskeläinen J, Voutilainen R. 2015. Premature adrenarche – a common condition with variable presentation. Horm Res Paediatr 83(4):221–31. https://doi.org/10.1159/000369458
Van den Bergh BRH, van den Heuvel MI, Lahti M, Braeken M, de Rooij SR, Entringer S, et al. 2020. Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neurosci Biobehav Rev 117:26–64. https://doi.org/10.1016/j.neubiorev.2017.07.003
Widén E, Silventoinen K, Sovio U, Ripatti S, Cousminer DL, Hartikainen A-L, et al. 2012. Pubertal timing and growth influences cardiometabolic risk factors in adult males and females. Diabetes Care 35(4):850–6. https://doi.org/10.2337/dc11-1365
Williams KM, Oberfield SE, Zhang C, McMahon DJ, Sopher AB. 2015. The Relationship of Metabolic Syndrome and Body Composition in Children with Premature Adrenarche: Is It Age Related? Horm Res Paediatr 84(6):401–07. https://doi.org/10.1159/000441498
Witchel SF, Pinto B, Burghard AC, Oberfield SE. 2020. Update on adrenarche. Curr Opin Pediatr 32(4):574–81. https://doi.org/10.1097/MOP.0000000000000928

