Available online at: https://doi.org/10.18778/1898-6773.87.2.02
 https://orcid.org/0009-0003-2596-7867
https://orcid.org/0009-0003-2596-7867
Biological Anthropology and Comparative Anatomy Research Unit, School of Biomedicine, The University of Adelaide, Frome Road, Adelaide, Australia
 https://orcid.org/0000-0002-9708-6220
https://orcid.org/0000-0002-9708-6220
Biological Anthropology and Comparative Anatomy Research Unit, School of Biomedicine, The University of Adelaide, Frome Road, Adelaide, Australia
Bachelor of Doctor Assistance Department, DDT College of Medicine, Gaborone, Botswana
 https://orcid.org/0000-0002-6388-0725
https://orcid.org/0000-0002-6388-0725
International Centre for Rock Art Dating, Hebei Normal University, Shijiazhuang, China
Independent Researcher
 https://orcid.org/0000-0003-1941-2286
https://orcid.org/0000-0003-1941-2286
Biological Anthropology and Comparative Anatomy Research Unit, School of Biomedicine, The University of Adelaide, Frome Road, Adelaide, Australia
Institute of Evolutionary Medicine, University of Zurich, Zürich, Switzerland
ABSTRACT: In this article we seek to integrate theories of music origins and dance with hominin fossil anatomy and the paleoecological contexts of hominin evolution. Based on the association between rhythm in music, dance and locomotion, we propose that early bipedal hominins may have evolved neurobiological substrates different from other great apes due to the rhythmic aspects of bipedal walking and running. Combined with the emancipation of the hands resulting from erect posture, we propose that the neurobiological changes necessary for technological innovation, cultural practices and human musical abilities may have evolved, at least in incipient form, much earlier than previously thought. The consequent ability to synchronize movement and sound production may have also proved beneficial as early bipedal hominins ventured out of late Miocene and early Pliocene woodland and forested habitats and into more open habitats with increased predation risk. We also postulate that, along with bipedalism, paedomorphic morphogenesis of the skull at the base of the hominin clade was a necessary prerequisite for the evolution of vocal modulation and singing in later varieties of hominin. To date research into the evolution of music and dance has yet to be integrated with the fossil and paleoecological evidence of early hominin evolution. This paper seeks to fill this lacuna in the extant literature on human evolution. We also suggest that autocatalytic feedback loops evolving synergistically with hominin erect posture, skull and hand morphology, neurochemical processes and the self-domestication syndrome, have been operative from early hominins some 6 Ma to the present. We document this process by reference to primatological, ethnographic, neurochemical and archaeological data.
KEY WORDS: evolution of music, evolution of dance, early hominins, bipedalism, paleoecology, locomotion, archaeology, Ar. ramidus, self-domestication.
In recent decades there has been a resurgence of interest in the evolutionary foundations of music and dance (Falk 2004; Brown et al. 2006; Mithen 2009; Brown 2017; Clark and Henneberg 2017; Dissanayake 2021; Benítez-Burraco and Nikolsky 2023). Various approaches have been adopted to elucidate the evolutionary origins and adaptive significance of such human behaviours. For example, based on evidence from chimpanzee rhythmic perception, it has been argued that the prerequisites for music and dance probably existed in the common ancestor shared by humans and chimpanzees (Hattori and Tomonaga 2020). In a similar vein, based on vocal tract anatomy and skull architecture, it has been proposed that early hominins evolved an anatomical configuration more conducive to musical vocalization than that evident in chimpanzees – and that consequently research into the evolutionary building blocks of music and language should focus on late Miocene and early Pliocene fossil anatomy (Clark and Henneberg 2017).
There has also been important research looking into the relationship between locomotion and rhythmic aspects of music and dance. This work has illuminated the degree to which the rhythmic aspects of walking may share neurobiological substrates with the rhythmic structuring of music and dance (Friberg and Sunberg 1999; Shove and Repp 1995; Thaut 2009; Bengtsson et al. 2009; Grahn and Rowe 2009; Thaut 2013). Additionally, it has been proposed that the evolution of the human bipedal locomotor adaptation gave rise to forms of metrically synchronised bodily entrainment using the upper and lower limbs, a form of entertainment that was to later form the basis of rhythmic vocal chorusing and music (Brown 2022).
While links have been made between the emergence of erect trunk bipedalism and the evolution of music and dance (Mithen 2009), very little research has explored this association in the context of emerging fossil and palaeoecological data regarding the emergence of erect bipedalism in early hominins. Given facultative bipedalism is believed to have emerged in early forest and woodland dwelling hominins such as Ar. ramidus, which then developed into obligate bipedalism among Australopiths (Lovejoy and Latimer et al. 2009; Kimbel et al. 2014), the association between music, dance and locomotory adaptations warrants further exploration in the context of emerging evidence from the fossil record.
The concept of self-domestication has been used to explore the origins of both language and music, highlighting associations with cultural transmission, pro-sociality and neurochemical regulation (Clark and Henneberg 2017; Thomas and Kirby 2018; Benítez-Burraco and Nikolsky 2023). Importantly, oxytocin is thought to be associated with in-group cohesion in chimpanzees and bonobos (Brooks et al. 2022) as well as the prosocial and affiliative aspects of music and dance in humans (Dissanayake 2021; Harvey 2020). While the oxytocin system is thought to be an important component of hominization and the evolution of cooperative and alloparental breeding systems (Hrdy 2009; Lovejoy 2009), it has yet to be fully explored in the context of the evolution of music, dance and relevant fossil and palaeoecological data. For example, while Clark and Henneberg (2017) analyse the possible relevance of the oxytocin system in explicating the evolutionary building blocks of music and language in early hominins such as Ar. ramidus, to date such an approach has not been explored in the context of early hominin paleoecology and the demographic expansion of the Australopithecines into non-arboreal habitats.
Archaeological approaches to the evolution of music have been discussed by numerous researchers (Blake and Cross 2008; Morley 2013; Fazenda et al. 2017; Turk et al. 2018). However, taphonomic issues may problematize such research as the earliest evidence for such behaviors may not have been preserved in the archeological record (Bednarik 1994). Despite such problems, putative musical instruments have been attributed to not only Homo sapiens but also Neanderthals (Turk et al. 2018). Additionally, caves possess acoustic properties that have long been exploited by humans (Fazenda et al. 2017). This behavioral phenomenon is very suggestive in light of emerging evidence of cave occupation by other hominin species in addition to Homo sapiens (Fuentes et al. 2023; Jaubert et al. 2016). For example, is it possible that occupation of caves by hominins may have contributed to an enhancement of auditory perception and sound production capabilities?
There has also been research exploring the previously overlooked sound producing properties of stone tools (Blake and Cross 2008). Increasing evidence pushing back the antiquity of tool manufacture to the Australopithecines (Harmand et al. 2015) suggests the possibility that early hominins were using physical objects to produce sound. Given chimpanzees use objects such as trees in such a manner (Eleuteri et al. 2022) it is possible that Australopiths may have used stone tools not only in procuring food but also to produce sound.
Based on their tool manufacturing abilities, it has been suggested Australopiths had elevated levels of hand motor control resulting from reorganization or expansion of the brain’s various motor regions (Harmand et al. 2015). Combined with enhanced manipulative abilities resulting from bipedalism, which enables emancipation of the forelimbs from locomotion (Lemelin and Schmitt 2016), it seems possible that Australopiths may have possessed sound production abilities beyond that evident in other great apes. While it has been previously suggested that stone tools may have been the first musical instruments (Montagu 2004), this theory has not been investigated in the context of early hominin anatomy, archaeology and paleoecology. Given the previously overlooked sound producing properties of stone tools it has been argued that existing lithic collections could be reanalysed in order to discover possible evidence of early human sound production (Blake and Cross 2008: 17).
It has also been argued that the collaborative synchronization underpinning music and dance may have evolved as a form of coalitionary signaling (Hagen and Bryant 2003: 24); additionally it has been proposed that such forms of coordinated sound production may have been utilized by hominins to deter predators, particularly as they moved out of arboreal habitats into more open habitats with higher predation risk (Jordania 2014, 2020). Importantly, one of the major shifts in hominin phylogeny was the demographic expansion of Australopithecines out of forested and woodland areas, and into more open and diverse habitats as obligate terrestrial bipeds (Meindl, Chaney, and Lovejoy 2018).
In this article we explore possible evidence for early forms of hominin sound production. We propose that as early hominins became obligate bipeds and moved out of arboreal forest and woodland habitats, the anatomical configuration associated with the skull, hands and body would have become part of an adaptive suite facilitating enhanced sound production using both the voice and objects. We propose that these abilities may have evolved as part of a broader adaptive complex involving enhanced levels of in-group cooperation. It was these early forms of sound production, and the associated changes in motor systems and neurobiological substrates, that formed the phylogenetic building blocks of later forms of communication, music, dance and ritual behaviors. We also suggest ways in which this hypothesis can be tested by increased awareness of other forms of sound production that may have left traces in the archeological record and which to date have been overlooked.
In this section we outline a model of human music, language, dance and sound production grounded in evolutionary percussors that constitute the putative phylogenetic building blocks of these forms of cultural expression. This will involve synthesizing data on paleoecology, predation pressure and hominin fossil anatomy. Importantly, we will develop a broader conception of sound production than that normally conceived of in modern cultures. For example, an evolutionary and cross species conception of sound production includes song, nonverbal vocalization, sound made with the body, physical substrates and manufactured objects – which in the case of past hominin cultures may include the sound producing properties of stone tools or the acoustically resonant spaces inside cave structures (Blake and Cross 2008, 2015; Fazenda et al. 2017). Many of these forms of sound production or acoustic signaling are embedded in ecological and cultural contexts in which auditory forms of sociality would have had survival benefits for those individuals or groups that possessed them (Blake and Cross 2015).
The unique features of hominin sound production, whether it be vocal, bodily or produced with objects, seem to be related to the unique hominin anatomical configuration. These include erect bipedalism, non-locomotory hand morphology, a flat face and loss of the canine honing complex characteristic of other primates. We will argue these traits, while not being sufficient, are nevertheless necessary preconditions for the evolution of the motor systems and neurobiological substrates that subserve vocal modulation, tool manufacture and the creation and use of musical instruments. We also explore evidence that obligate bipedalism led to the evolution of neurobiological systems that subserve not only the pace and timing of locomotion, but also rhythmic perception necessary for the temporal structuring of music and dance (Thaut 2013). Given these anatomical features are very ancient we explore their original emergence and their important role in hominization.
Researchers have argued that synapomorphic traits that differentiate hominins from other great apes include a more anteriorly positioned and horizontally oriented foramen magnum associated with vertical neck posture in orthograde bipedal locomotion, as well as dental traits such as reduced canines (Mongle et al. 2019). Importantly, a recent analysis of 300 fossils spanning 6 million years has, according to the authors, demonstrated that ‘male canine size reduction occurred early in human evolution, broadly coincident with the adoption of bipedality’ (Suwa et al. 2021: 1). Importantly, a centrally postioned foramen magnum characteristic of hominin bipedalism, combined with reduced facial projection and lack of aggressive canine armory, gives rise to the unique hominin skull and vocal tract configuration that is a necessary prerequisite for the evolution of spoken language and singing (Clark and Henneberg 2017).
The majority of Miocene hominoids are believed to have been arboreal or semi-arboreal (Rose 1993). Additionally, there is evidence that early hominins were arboreal facultative bipeds (Lovejoy and Simpson et al. 2009). It therefore seems likely that early hominins sought refuge from predators in trees – as is the case in other primates (Baldwin et al. 1981: 482; Hamilton 1982: Boesch 1991: 228; Iwamoto et al. 1996: 393). In addition to such ecological factors, there is also a vocal component to primate predator defence involving loud vocalisations and alarm calls (Tutin et al. 1981; Tsukahara 1993; Boesch 2009: 22–23 and 52–53). There may also be predation induced selection on social structure associated with non-arboreal habitats; for example, savanna-living chimpanzees travel in large numbers when moving between patches of trees, which may be an anti-predator response (Tsukahara 1993) while baboons who evolved in open savanna or semidesert habitats jointly drive predators from the group (Kummer 1967: 154–155). Additionally, geladas who also inhabit more open habitats, utter alarm calls and form multilevel fission‐fusion social structures in response to predation – which has been proposed as a possible model for early hominin occupation of non-arboreal habitats (Lin et al. 2020).
What are the implications of the above observations for the evolution of hominin sociality and sound production? We suggest that when erect bipedal hominins moved out of woodland and forested ecologies into more open habitats, they may have also used vocalisation, alarm calls and various forms of non-vocal sound production to deter predators. Importantly, it has been argued that hominins may have been subject to predation, from Ardipithecus ramidus to modern humans, and that consequently social cooperation and associated neuroendocrine systems may have evolved as a response to early hominins being initially a prey species – a theory that finds support from evidence that cooperation can reduce lethal outcomes resulting from predator attacks (Hart and Sussman 2011, 2019). We would add to this observation the possible role synchronised sound production and movement may have played in this putative adaptive suite.
Many of the features we analyze in early arboreal facultative bipeds such as Ar. ramidus seem to be associated with forested and woodland ecologies. Evidence for this view is based on paleohabitat and dentition (White et al. 2009). While this position has been questioned based on the existence of contemporaneous ‘tree or bush savanna’ in the region (Cerling et al. 2010), White and colleagues assert that Ar. ramidus did not occupy such an ecological niche, with the species' primary habitat consisting of closed forest and woodland (White et al. 2010). It has also been argued that Ar. ramidus may not be a hominin but a fossil relative of chimpanzees – and that additionally there is little evidence of bipedalism in the species (Senut 2015: 2053–2054). This perspective, however, seems at odds with a more recent analysis of character sampling that suggests Ar. ramidus was in fact a basal hominin (Mongle et al. 2019). This position is further supported by evidence of facultative bipedality in the Ar. ramidus cranial base, which is believed to show extensive affinities with Australopithecus and Homo (Kimbel et al. 2014). Further, the cranial base angle, as well as facial, jaw and canine morphology in Ar. ramidus are distinctly different from chimpanzees, showing pronounced affinities with hominins (Clark and Henneberg 2015, 2017). Importantly, erect bipedalism also means hominins do not have hands specialized for locomotion as in quadrupedal apes. It is this lack of specialization in the forelimbs that ultimately underpins human technological and cultural evolution as well as coevolutionary processes between the hands and the brain (Lundborg 2013).
The absence of large aggressive canines and canine sexual dimorphism in hominins has long puzzled evolutionary theorists and numerous explanations have been put froward to account for this unusual phenomenon. For example, in The Descent of Man Darwin argued that with the invention of clubs and other weapons, canines were no longer necessary in male on male conflict (Darwin 2004:73). However, this does not seem to be a complete solution for it does not postulate a selective mechanism for canine reduction; it merely suggests weapons could have taken the place of canines without explaining what potential selective pressures may have been involved in canine reduction.
It has been suggested that selection for the diminution of aggression and changes in hormonal regulation may have been a possible evolutionary mechanism resulting in canine reduction (Holloway 1967). Others have proposed similar solutions to the dilemma of canine reduction with a shift in social and mating behavior and associated neurochemical regulation being considered a possible explanation (Lovejoy 2009; Clark and Henneberg 2017, 2015). Some researchers have suggested that this may have involved a socio-behavioural shift that minimized male–male aggression possibly mediated by female mate choice (Suwa et al. 2021). What is significant is that early hominins such as Ar. ramidus seem to have lost the aggressive canine armory characteristic of many other primate species prior to becoming terrestrial obligate bipeds. It is important to note that Ar. ramidus still possessed a grasping big toe and that the species most likely climbed and walked bipedally among the large trees of late Miocene and early Pliocene Africa (Lovejoy and Simpson et al. 2009; Lovejoy and Latimer et al. 2009).
Importantly, some species such as baboons evolved extreme forms of canine sexual dimorphism, which are in part related to the need for predator defense in what are essentially hostile savanna habitats with high predation risk (Plavcan and van Schaik 1992). Having lost aggressive canine armory in forested and woodland habitats, the question arises as to how early hominins would have survived when they ventured into habitats lacking trees that could serve as places of refuge from predators? Is it possible that coordinated sound production and body movement were part of an antipredator adaptive suite?
Based on the above observations, we hypothesize that early hominins such as Ar. ramidus may have already possessed social adaptations that enabled significant levels of prosocial coordination and cooperation. While such adaptations no doubt would have evolved for many reasons, they would have been useful when encountering predators, and such encounters may have been one component in a complex selective regime favoring their enhancement. Consequently such cooperation, combined with synchronized vocalization and sound production may have manifested itself in a form of “territorial chorus” that provided some of the building blocks for the evolution of music and language (Brown 2017). Our argument is premised on the notion that pro-sociality and cooperative breeding correlate across primate taxa with reduced canine size and reduced canine sexual dimorphism – social adaptations that may explain such reduction in early hominins (Lovejoy 2009; Clark and Henneberg 2015). Importantly, co-operatively breeding primates show elevated levels of generalized pro-social behavior (Burkart et al. 2007) as well as coordinated and synchronized vocalization abilities akin to human conversation (Choi et al. 2015). This has led to speculation that co-operative breeding in Ar. ramidus may have been accompanied by increased vocal synchrony and ability to modulate vocalizations – an assertion that finds additional support from the species skull architecture and vocal tract anatomy which is more human-like when compared with chimpanzees (Clark and Henneberg 2017).
Significantly, as noted by Schruth and colleagues among anthropoids only the monogamous and swinging lesser apes seem to share the human aptitude for spectral musicality (Schruth et al. 2023). Consequently, given the association between such aptitudes and forest dwelling arboreal primates, they propose musical ability as a primitive trait characteristic of Miocene apes that was consequently lost, or which became atrophied as primate species ventured into more terrestrial habitats. Humans are unusual among terrestrial primates in that we retain such musical albitites, which are much more common in arboreal species. As the authors suggest in ‘strictly terrestrial primates, previously evolved associations between musical calling and [arboreal] locomotion appear to have atrophied’ (Schruth et al. 2023: 9). This may be due to the fact that singing in open terrestrial habitats many attract predators, whereas this may not be an issue in forest canopies which serve as refugia (Jordania 2020). From such a perspective, as opposed to losing such albitites when they ventured into more terrestrial habitats, hominins retained them, which then served as the phylogenetic building blocks for later forms of musicality. The question that needs addressing is why did terrestrial hominins retain musical calling?
The association between arboreal forest ecologies, monogamy and protomusical calling noted by Schruth and colleagues, is also very suggestive in the context of early hominin social and mating behaviour. It suggests that early hominins such as Ar. ramidus may have not only been anatomically similar to arboreal lesser apes (Lovejoy 2009) but may also have shared with such species aspects of mating, social and musical behavior (Schruth et al. 2023: 9; Clark and Henneberg 2017). This may have involved pair-ponding and forms of cooperative or alloparental care of offspring (Lovejoy 2009 ) possibly mediated by forms of prosocial vocal synchrony (Clark and Henneberg 2017).
While there is extensive evidence of cooperative breeding and allomaternal care in Homo sapiens (Hrdy 2009) it is unclear when this breeding strategy evolved. Consequently, it is very difficult to infer social and mating systems from fossil anatomy with any certainty. However, reduced canine size and dimorphism does correlate across primate taxa with reduced male on male aggression and Ar. ramidus canine size and dimorphism is nearly human-like; consequently, it has been argued this evolutionary pattern in Ar. ramidus indicates a ‘profound behavioural shift associated with comparatively weak levels of male aggression early in human evolution, a pattern that was subsequently shared by Australopithecus and Homo (Suwa et al. 2021: 1). Such an adaptive complex in Ar. ramidus may have involved cooperative breeding and male investment in the maternal metabolic budget as an alternative reproductive strategy to male on male tournament behaviour (Clark and Henneberg 2015). This may mean that the system of cooperative breeding that is believed to have facilitated the increase in brain size in the Homo lineage (Isler and Schaik 2012a) may have evolved much earlier at the base of the hominin clade (Lovejoy 2009; Clark and Henneberg 2015). This position finds support in the fact that cooperatively breeding primates such as marmosets, who engage in forms of vocal communication similar to humans, have a low encephalisation quotient (Ghazanfar and Takahashi 2014) and that human co-operation is not dependent on advanced cognitive abilities, nor on large brain size, and that therefore human pro-sociality may have evolved before the emergence of large brained hominins (Isler and Van Schaik, 2012a).
Additionally, it has been suggested that increases in body and brain size in excess of earlier Australopithecines, would have required contribution to the infant metabolic budget by individuals other than the mother – and that consequently cooperative or alloparental care provides a plausible explanation for the increase in brain size in the Homo lineage (Hrdy 2009; Isler and Van Schaik 2012b). From this perspective, “emotional modernity” including cooperative breeding, uniquely human forms of mind reading and intersubjectivity, may have evolved long before increases in brain and body size – in fact such adaptations may have been necessary for such evolutionary trends, which require increased metabolic allocation to infant and childhood growth from other individuals in addition to the mother (Hrdy 2009).
One of the important issues related to the adoption of obligate erect bipedalism in the Homo genus, is that it may have facilitated increased metabolic allocation to infant growth and a slower pace of development for infants resulting from the need to learn more complex tool use and foraging technologies (Potts 2011). Significantly, based on correlations between brain size and life history trajectories (Smith and Tompkins 1995) it has been argued that Ar. ramidus life history was similar to chimpanzees (Clark and Henneberg 2015). As brain and body size increased in Homo erectus, it has been proposed that life history was slowed down to include extended childhood and adolescent phases of development during which social learning could occur, thus enabling the acquisition of language skills (Locke and Bogin 2006) as well as cultural competencies such as increasingly complex tool manufacture (Bogin 2003; Sterelny 2012). Such changes in life history may have underpinned the socio-behavioural adaptations contributing to the demographic expansion of Homo erectus (Hawkes and Coxworth 2013; Hrdy 2009; O’Connell et al. 1999). We would add to this adaptive complex the important role of music and dance in the social transmission of cultural knowledge relating to hunting and kinship systems; for example, in extant gathering and hunting cultures music and dance are the primary means of transmitting knowledge regarding waterholes and hunting grounds from one generation to the next – transmission that is particularly important during adolescent initiation rites (Strehlow 1971; Stanner 2014).
We suggest that the building blocks of this adaptive suite, at least in incipient form, may have been in place at the base of the hominin clade – an inference based on the above mentioned reduction in both canine size and dimorphism (Lovejoy 2009). Significantly, it has been argued that reduced canine and body size sexual dimorphism in hominins suggests that co-operative breeding and allo-parental care may have provided the social contexts for language evolution to develop – and particularly the acquisition of language abilities by infants and juveniles due to intensified interaction with numerous carers (Fitch 2007). We seek to extend this observation in our analysis of the evolution of coordinated sound production and bodily movement. For example, if cooperative breeding did evolve in early small brained hominins, is it possible that vocal cooperation of the kind we find in other cooperatively breeding primates was part of this adaptive complex? And could such cooperation and synchronised sound production have formed the early phylogenetic building blocks upon which later forms of music and dance were built?
The thesis outlined above is obviously a tentative hypothesis and other scenarios are possible. For example, others have argued, based on measures of bodily size sexual dimorphism, that early hominins evolved a gorilla-like polygynous mating system, and that this consequently evolved into human social and mating systems (Geary, Bailey, and Oxford 2011). This approach, while convincing on some grounds, does not account for why early hominins lost the large aggressive canine complex – additionally it has been argued that sexual dimorphism in early hominins falls within the range of modern humans (Reno and Lovejoy 2015). This debate is still ongoing and inferences from fossil anatomy to social and mating systems remain highly contested. In this paper we adopt, as a working hypothesis, the theory that reduced male on male aggression as evidenced by loss of large aggressive canines signals an important behavioural shift involving elevated levels of pro-sociality and possibly forms of alloparental care of offspring – including male investment in the maternal metabolic budget (Clark and Henneberg 2015). We also suggest that adaptations such as increased levels of pro-sociality in Ar. ramidus would have benefited later hominin species such as the Australopithecines when they ventured out into more open nonarboreal habitats with increased predation risk.
More specifically, we suggest that coordinated group behavior involving synchronized vocalization, synchronized sound production using physical objects or substrates, in addition to synchronized body movements, could have deterred predators (Jordania 2009, 2020). Because hominins had already developed cooperative social structures in forested and wooded ecologies they could consequently cope with high predation risk in more open nonarboreal habitats. These adaptations, we suggest, were exapted as the climate changed and the shrinkage of the great forest ecosystems of the Miocene meant that great apes and hominins needed to rely more and more on forms of terrestrial locomotion – with great apes adopting quadrupedal knuckle walking and hominins obligate erect bipedalism. In this sense both erect bipedality and pro-sociality in Ar. ramidus were necessary pre-requisites of the Australopith demographic expansion as obligate terrestrial bipeds.
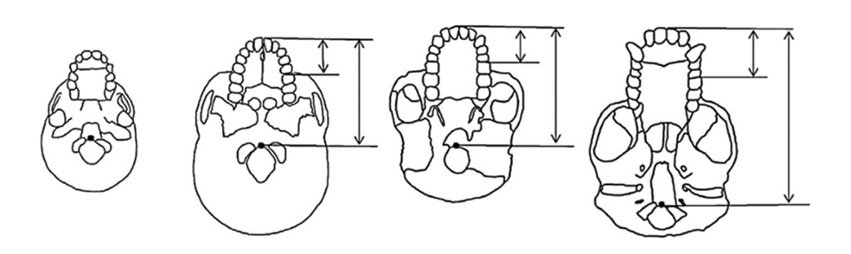
In this section we propose that erect trunk orientation and the associated skull and hand morphology have much greater phylogenetic depth than traditionally supposed. There are two important consequences of erect trunk orientation that relate directly to the origins of vocal abilities such as language and singing, as well the motor control necessary for using objects to create sequentially structured rhythm. Firstly bipedalism, due to where the spinal cord enters the skull and connects with the brain, contributes to the shortening of the horizontal vocal tract, with such shortening being a necessary precondition for the evolution of human language and singing (Figs 1 and 2). Secondly, obligate bipedal locomotion emancipates the forelimbs from locomotion (Lemelin and Schmitt 2016). This is the crucial factor underpinning the evolution of hominin hand morphology that enables the development of precision grasping and the consequent co-evolution between the brain and the hands that underpins human technological and cultural accomplishments – from the earliest stone tool industries to classical piano playing (Lundborg 2013; Wilson 1999). We also speculate that the creation of such physical objects out of stones or other materials would have provided hominins with additional means of creating sound that could potentially deter predators – that is an evolutionary precursor of “drumming” (Jordania 2009).

Fig. 1. Length of the face, palate and horizontal vocal tract. This figure graphically illustrates the differences between chimpanzee and Ar. ramidus skull architecture. Note the more posterior position of the foramen magnum and basion (black dot) in the adult chimpanzee and the greater resemblance of both humans and Ar. ramidus to the infant chimpanzees. From left: infant chimpanzee, adult human, Ar. ramidus and adult chimpanzee. From Clark and Henneberg (2017)
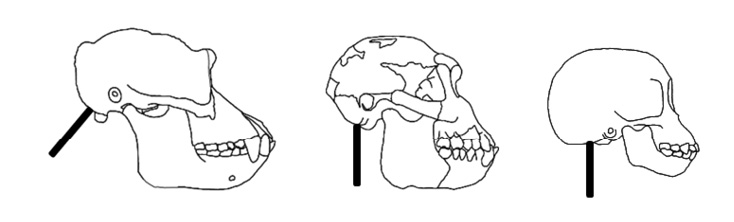
Fig. 2. Skull shape and facial projection. Note the spinal cord (black bar) enters from the bottom of the skull in Ar. ramidus and the infant chimpanzee and from the rear in the adult chimpanzee. This feature, in addition to reduced facial projection, suggests the evolution of paedomorphic skull morphogenesis in Ar. ramidus. From left: adult chimpanzee, Ar. ramidus and infant chimpanzee. From Clark and Henneberg (2017)
A crucial perspective we explore in this article is that the skull anatomy of non-human great apes, and particularly that associated with quadrupedal knuckle walking, represents an obstacle that prevents the evolution of human-like vocal ability – and that the absence of this obstacle in early hominins opened the way for language and vocal ability to evolve (Clark and Henneberg 2017). As already noted, many researchers have suggested that bipedalism evolved much earlier than traditionally supposed, with some arguing unique features of the hominin lineage may have their origin in Miocene and Pliocene ecological contexts. For example, there seems to be evidence of bipedal locomotor morphology in the European Miocene, which has been postulated as the ancestral form from which both humans and great apes evolved (Böhme et al. 2019). Other researchers have argued that hominin bipedalism and forelimb morphology may be primitive, with chimpanzee anatomy associated with quadrupedal knuckle walking being derived (Lovejoy et al. 2009; White et al. 2015). This view is consistent with evidence that humans did not evolve from a knuckle walking ancestor as previously assumed and that human bipedalism originally evolved as an adaptation to arboreal habitats (Kivell and Schmitt 2009). Others, on the other hand, have questioned the above interpretation, arguing that the last common ancestor humans share with great apes was in fact similar to chimpanzees in terms of possessing knuckle walking quadrupedal locomotor morphology (Chaney et al. 2022; Prang 2019; Prang 2021; Williams et al. 2023).
Whatever the case regarding the last common ancestor hominins share with chimpanzees and bonobos, there seems to be a consensus regarding the common ancestor of hominins. For example, mounting evidence from the Miocene and early Pliocene, suggests that the common ancestor of early hominins such as Ardipithecus, Orrorin, and Sahelanthropus was likely adapted to vertical climbing and perhaps suspension – and that the common ancestor of hominins was orthograde and not a knuckle walking terrestrial quadruped (Ward 2013).
Importantly, the grasping toe in Ar. ramidus was ‘retained for several million years on a foot otherwise adapted for terrestrial bipedalism’ (Williams et al. 2022: 71). This suggests, that in hominins at least, an early form of bipedal posture existed in conjunction with arboreal, climbing adaptations in the foot. The occurrence of erect facultative bipedality in forest and woodland dwelling hominins capable of tree climbing, is further supported by the cranial base and position of the foramen magnum in Ar. ramidus, which is similar to other bipedal hominins and distinct from other great apes (Kimbel et al. 2014).
It has been argued that bodily based metric entertainment amongst members of a social group may have evolved in the hominin lineage before the metrical elements of vocal musicality (Brown 2022). This theory is based on the observation that bodily and haptic based coupling in chimpanzees occurs more frequently when locomoting in an upright bipedal posture (Lameira, Eerola and Ravignani 2019). The implication of these findings is that early erect bipedal hominins such as Ar. ramidus may have had increased capacity for bodily based coupling and entrainment than other great apes. If so this would mean the building blocks of synchronized bodily movement (that is the phylogenetic precursors of dance) may have existed at least in an incipient form in early bipedal hominins. Consequent to this stage, such ability to metrically synchronize bodily movements would have formed the basis of metrical entrainment of vocalizations and the consequent evolution of synchronized and rhythmically structured group singing (Brown 2022).
If the above scenario is correct, it would mean the ability to synchronise body movements may have conferred evolutionary benefits on erect bipedal hominins as they ventured out of forested and woodland ecologies and into more open habitats. Consequently, when thinking about the emergence of obligate bipedalism the question that perhaps should be asked is “why did early hominins remain orthograde when they began exploiting terrestrial niches?” (Ward 2013: 1379). We could also add to this observation and ask what were the socio-behavioural and neurochemical factors associated with remaining orthograde and exploring terrestrial niches as erect trunk obligate bipeds? And what is the relevance of this anatomical configuration in subserving the neurobiological substrates of communicative capacities such as music and dance?
As already mentioned, the important point to emphasize when considering hominin brain evolution is that bipedalism emancipates the forelimbs and hands from locomotion. The consequences of this are that early bipedal hominins lacked the specialized wrist and hand morphology evident in chimpanzees, which prevents these apes from evolving complex manipulative abilities. It has been argued that retaining a more generalized configuration more conducive to precision grasping is what facilitated the coevolution between the brain and the hands characteristic of the hominin lineage (Lemelin and Schmitt 2016; Lundborg 2013; Napier 1993, 1962). Additionally, it has been suggested that neural reorganization and expansion of motor regions associated with hand motor control may have emerged with the Australopithecines (Harmand et al. 2015). It is these developments associated with bipedal locomotion and hand morphology that may have signaled the beginning of a unique form of neurochemical regulation involved in motor control – that is upregulation of the dopaminergic system. We explore this issue further later in this article.
The relationship between vocal abilities, motor control and movement have been extensively explored (Fitch 2011; Feenders et al. 2008; Lieberman 2009). For example, it has been argued erect bipedal locomotion provided the initial selective force for the enhancement of the subcortical sequencing ability involved in both motor control and cognition (Lieberman, 2009). Consequently, it has been asserted that the selective pressures that resulted in the evolution of the sequential processing necessary for tool manufacture, as well as human speech ‘may ultimately derive from upright bipedal locomotion, the initial hominid adaptation’ (Lieberman, 2009: 143 and 151). There is also significant evidence of analogies between motion and music, with researchers finding links between music and the force patterns associated with walking as well as relationships between physical motion and musical tempo (Friberg and Sunberg 1999; Shove and Repp 1995). Additionally, the ‘felt pulse’ patterns involved in locomotion and rhythmic perception are both believed to be based on the entrainment of oscillator circuits in the brain (Thaut 2013: 7 and 9).
Importantly, areas involved in rhythmic perception are related to those that regulate movement; for example cortico-cerebellar circuits that subserve conscious and subconscious responses to temporal structure are involved in rhythmic synchronization and rhythmic motor control (Thaut 2009), while music has been found to activate motor and premotor cortices that are not part of the classical auditory system of the temporal lobe (Bengtsson et al. 2009). Additionally, the basal ganglia shows a specific response to beats during rhythm perception, with a cortico-subcortical network and coupling of motor and auditory areas being associated with musicality (Grahn and Rowe 2009). This association is also supported by evidence that listeners often experience music as a type of virtual movement analogous to physical motion, and that during ontogeny mastering melodic leaps and steps accompanies learning to walk, and that during childhood play, musical patterns are often associated with the affective characteristics of the accompanying locomotion (Nikolsky 2023).
Significantly, in animals that have high levels of vocal learning, spontaneous rhythmic movement to auditory rhythms seems to be more common than in species that lack such learning. Additionally in high vocal learners, motor planning regions are in tight reciprocal communication with forebrain auditory regions throughout life, suggesting that vocal learning may have been a preadaptation for the evolution of human beat perception and synchronisation (Patel 2021). This perspective seems to differ from Brown’s thesis discussed above that bipedal bodily based synchronisation and metric entrainment were evolutionary precursors to the metrical and melodical aspects of music (Brown 2022). While postulating vocal learning as a preadaptation for spontaneous rhythmic movement to auditory rhythm is different in emphasis from seeing bodily synchronization as an evolutionary precursor to synchronised metrical singing, both theories do link the evolution of rhythmic perception with vocalisation and melodic vocal synchrony. The difference between the two approaches is which has evolutionary priority – vocal learning or bodily based synchronized entrainment?
In terms of the model outlined in this paper the question that arises from the above discussion is: did vocal learning in early hominins such Ar. ramidus and the Australopithecines evolve prior to the evolution of beat perception? Related to this question is the evolutionary priority of the hominin vocal tract facilitating vocalization and language relative to obligate erect bipedalism. In terms of the issues discussed above it is important to emphasize that we see a shift to a human-like vocal tract in Ar. ramidus long before we see the emergence of obligate terrestrial bipedalism in the Australopithecines. Does this imply that vocal learning, may have preceded the kind of bodily based rhythmic entrainment associated with bipedalism? Or did, as intimated by Brown’s model, the emergence of obligate bipedalism in Australopithecines form the evolutionary basis of later forms of vocally based metical synchronization – forms of synchronization that were absent in Ar. ramidus?
Resolving these issues with any certainty is far beyond the purview of this essay. However, what our model of sound production informed by ecological and fossil data provides is a framework for thinking about which aspects of the modern adaptive suite may have had evolutionary priority. What is clear is rhythmic perception associated both with locomotion and music seem to be linked. The question is which components of this aspect of the modern human adaptive suite have the greatest phylogenetic depth? That is, did vocal learning give rise to beat perception or are the metrical aspects of musical rhythm products of locomotor adaptations? As we speculate below, given early hominins may have been forest and woodland dwelling singers before becoming obligate bipeds, could this mean that vocal learning had priority in the evolution of beat perception and synchronization?
The link between musical perception and locomotion is also suggested by research findings that patients with Parkinson’s disease, or who have suffered strokes or traumatic brain injury, benefit from rhythmic auditory stimulation (Thaut et al. 1997; Hurt et al. 1998; Thaut et al. 2001; Thaut 2013). Significantly, Parkinson’s disease is a neurological disorder involving the progressive degeneration of the dopaminergic system (Raglio 2015), a system which is believed to be central to the emergence of obligate terrestrial bipedalism in the genus Homo (Previc 2009). Based on such findings, it has been suggested that the ability to maintain an internal rhythm associated with bipedal locomotion and fluid walking, would have spread over into a capability for maintaining rhythmic sound – and the associated freeing of the arms, the hands, and the upper torso, in addition to enhanced muscular control may have underpinned the evolution of dance within the Homo genus (Mithen 2009).
What the above discussion suggests is that hominins have unique neurobiological adaptations associated with entrainment to an external pulse, and that this ability to rhythmically structure sound and movement is related to bipedal locomotion. Given obligate bipedalism seems to be very ancient it is reasonable to suppose that early hominins such as the Australopithecines possessed a form of rhythmic sound production and perception that may have been more advanced than in other great apes. We suggest such capacity for rhythmic sound and movement associated with bipedal locomotion may have been crucial to the collaborative synchronization underpinning music and dance based coalitionary signaling (Hagen and Bryant 2003: 24). Further, such an adaptation may have been important not only in signaling between groups of hominins, but also during interactions with predators. In what follows we flesh out these ideas in more detail and how they relate to the demographic expansion of Australopithecines.
In the following we assume Ar. ramidus to be ancestral to Australopithecus. (White et al. 2015; White et al. 2009; Kimbel et al. 2014). More specifically, we suggest as a working hypothesis that elevated levels of pro-sociality, and possibly cooperative breeding or alloparental care of offspring, were adaptations already in place in Ar. ramidus and that these adaptations were necessary for the successful demographic expansion of Australopithecus. As already noted Australopithecus shares with earlier hominins such as Ar. ramidus a centrally positioned foramen magnum as well as loss of the aggressive canine armory characteristic of other primates (Suwa et al. 2009; Kimbel et al. 2014; White et al. 2015). However, they possess adaptations suggestive of a greater degree of terrestrial bipedalism than earlier forest and woodland dwelling hominins. It is this adaptation that is believed to be associated with their demographic expansion into more diverse habitats than those occupied by earlier hominins (Meindl, Chaney, and Lovejoy 2018).
Significantly, early hominins such as O. tugenesis and Ar. ramidus may have avoided predators through cryptic habitation in forests (Treves and Palmqvist 2007). However, having expanded into nonarboreal habitats, it is likely that Australopithecus became subject to increased predation pressure – an observation supported by extensive analysis of the fossil remains of this genus (Brain 1983). Significantly, as a result of predation pressure, it has been argued that hominins inhabiting Pliocene African savanna-woodlands might have engaged in more visual, and possibly auditory vigilance, than those living in closed forested habitats (Treves and Palmqvist 2007: 367).
The evolution of African carnivores coincided with a decrease in woodland relative to grassland with the consequence that the type and number of carnivores changed throughout the course of hominin evolution. For example, between 6 and 3.6 Ma there were five genera of large carnivores without extant analogues, and from the mid-Pliocene (3.6 Ma) these groups were joined by an additional eight new genera of carnivores (Treves and Palmqvist 2007: 357). Additionally, from 1.8 Ma onward archaic carnivores went extinct in Africa, partly as a result of a global carnivore guild turnover and species replacement. The important issue here for the adoption of obligate erect trunk bipedality are the significant number of predators that existed between 3.6 and 1.8 Ma – a period which coincided with the Australopithecus demographic expansion into nonarboreal habitats. As Terves and Plamqvist write:
Given the existence of numerous ambush predators between 3.6–1.8 Ma, hominins would have experienced strong selection for efficient vigilance. Large parties of apes organized like those of chimpanzees are conspicuous and costly in terms of individual vigilance, competition for food and agonistic social interactions, hence we propose early hominin foraging parties would have adopted more cohesive and calmer social organization to maintain efficient vigilance and reduce conspicuousness to carnivores during diurnal foraging. Groups formed of trusted and familiar individuals often forage and travel with high levels of interindividual proximity, experience minimal conflict, and coordinate vigilance more easily.(Treves and Palmqvist 2007: 370)
One of the reasons postulated for the absence of singing in the majority of terrestrial primates is that singing will attract the attention of ground dwelling predators (Jordania 2020). Humans are an exception to this trend being one of the few singing terrestrial primates (Schruth et al. 2024). Is it possible that early hominins retained singing from their arboreal ancestors with one of its uses being an anti-predator adaptation? In this sense, far from singing attracting predators it may have been, along with high levels of interindividual proximity and coordinated vigilance, an effective means of deterring them and consequently reducing mortality risk.
It has been argued that the demographic shift of Australopiths into nonarboreal habitats necessitated a change in social structure to one unlike that evident in any other extant non-human great apes (Meindl, Chaney, and Lovejoy 2018). For example, such expansion into nonarboreal habitats would have exposed young and adult females to high levels of extrinsic mortality due to predation. However, if the early hominin social structure was one based on allo-parental care, reduced intragroup conflict, group level cooperation, and male forms of group protection and investment, then female survivorship could be enhanced both before and after sexual maturity, leading to population increase and demographic expansion (Meindl, Chaney, and Lovejoy 2018).
Importantly, large groups of animals are more likely to encounter a predator, but less likely to be attacked by it, which may result from inherent benefits of group living and cooperative breeding systems (Sorato et al. 2012). Further, given cooperative defence and shelter construction are some of the primary benefits of sociality, it has been argued that predation risk may be fundamental for the transition toward complex social organization (Groenewoud et al. 2016). Additionally, numerous animals engage in coalitionary vocal signaling; for example female lions roaring in chorus deters alien and potentially infanticidal males, gibbons use communal screaming to defend group boundaries and repel predators (Hagen and Bryant 2003: 26–27) while chimpanzees employ group level co-operation mediated by vocal calls as a means of predator defense (Boesch 2009: 22–23 and 52–53). Additionally, baboons jointly drive predators from the group (Kummer 1967: 154–155) while geladas utter alarm calls and seem to form multilevel fission‐fusion social structures in response to predation (Lin et al. 2020).
Support for possible predation on early hominins comes from the Swartkrans deposits, which suggest Australopiths may have been attacked while in the caves, a distinct possibility given that carnivores also use such caves as dens (Brain 1994; Treves and Palmqvist 2007: 363). Given the likelihood of predation pressure on Australopiths, then what role would have synchronized vocalization and sound production played as they expanded out of forest and woodland ecologies and out into diverse non-arboreal habitats? In the absence of aggressive canine armory, is it conceivable that early hominins used synchronized sound production and body movement to deter predators?
When dealing with this issue it is important to acknowledge that early forms of sound production may not have only been vocal but may also have been produced by the body, such as stomping on the ground or clapping, or by hitting physical objects together. Similar to humans, many other animals produce both vocal and somatic sounds. For example many mammals communicate non-verbally by drumming on their body or a substrate in order to attract mates, signal to predators or to establish territorial ownership – signals which may be a ritualization of phylogenetically older behaviors associated with running or digging (Randall 2015).
In gorilla’s such non-vocal sound production is evident in chest beating which is believed to convey information about size and competitive ability (Wright et al. 2021). Chimpanzees produce resonant sound using their body by moving one external object against another such as throwing rocks at tree trunks (Kalan et al. 2019). They also drum on trees which enables them to communicate long distances (Eleuteri et al. 2022) and at times such drumming may be integrated with vocalization such as the pan hoot (Arcadi, Robert, and Boesch 1998). Chimpanzees have also been observed performing so-called “rain dances” (Whiten et al. 1999), they show evidence of rhythmic swaying induced by sound (Hattori and Tomonaga 2020) they are able to synchronise their movements to an auditory rhythm (Hattori, Tomonaga, and Matsuzawa 2013) in addition to exhibiting spontaneous whole-body entrainment between two peers, suggesting possible empirical evidence for the phylogeny of human dance (Lameira, Eerola, and Ravignani 2019). Based on these findings it has been suggested the prerequisites for music and dance are deeply rooted in hominoid phylogeny and probably existed in the common ancestor shared by humans and chimpanzees approximately 6 million years ago (Hattori and Tomonaga 2020).
Elaborating on these observations it has been argued that such bodily and ‘haptic coupling may have been the earliest means for producing rhythmic entrainment between two agents in non-human primates.’ Additionally, given such behaviour is restricted to the bipedal manner of locomotion in chimpanzees, such studies ‘might have implications for the evolution of both bipedalism and dance’ (Brown 2022: 9). Given that Ar. ramidus was a facultative biped we can speculate that such haptic based locomotor coupling may have been more common than in chimpanzees who are infrequent bipedalists, with a locomotor anatomy designed for quadrupedal knuckle walking. This is even more so with the emergence of obligate bipedalism in the Australopithecines.
Given the ubiquity of sound production using the body and objects in mammals and primates, we can assume with some justification that a variation on such forms of communication existed in Ar. ramidus and Australopithecus. However, the unique anatomy of these early hominins would suggest that such abilities may have been much more complex than in other primates. For example, Australopithecus hand proportions are human-like primarily because of the unique locomotor adaptation of hominins (Almécija, Moyà-Solà, and Alba 2010). Importantly, greater manipulative capacity of Australopithecus relative to other great apes is suggested by Lomekwi stone technologies, which include hammers, anvils and sharp-edged flakes, which have been dated to 3.3 Ma (Harmand et al. 2015). Additionally there is evidence of stone-tool-assisted consumption of ungulates by Australopithecus afarensis (McPherron et al. 2010). Lomekwi technologies suggest that their makers had elevated levels of hand motor control resulting from reorganization or expansion of the brain’s various motor regions – and that such reorganization could have occurred before 3.3 Ma (Harmand et al. 2015). It is also possible that these adaptations provided the social niche in which selection for upregulation of neurochemicals associated with complex sequential processing and motor control may have occurred – as we explore in more detail below.
If the co-evolution of the brain, hands and motor systems had developed in Australopithecus to the point where these small-brained hominins were able to create hammers and anvils and use stone tools to butcher ungulates, then the question arises as to what other uses such objects and abilities were put? It has been proposed that the rhythmic percussive pattern produced by hitting two stones together to make tools may have been the world’s first musical instrument; for example ‘two flint knappers, chipping in ear-shot of each other, could have been the first musicians to produce rhythmic counter point as they interlocked their rhythms’ (Montagu 2004: 171). It has also been argued that hominins would have been aware of the acoustic properties of flint knapping and it is possible that they would have exploited such properties for communicative and social purposes (Blake and Cross 2008).
Concurring with the above authors we propose that the long sequence of strikes required to make such tools, the hitting of stones together, and the use of anvils, have acoustic properties that would most likely have appealed to early hominins. Would they have taken pleasure in the rhythmic structure of repeated blows to an object? Would they have used such objects to create sound in a similar manner to chimpanzees throwing rocks at or drumming on trees? Importantly, hard rocks, such as basalt, flint or chert, suitable, and actually used in the Lower Paleolithic for production of weapons and tools with sharp cutting edges and fine points, due to their crystalline nature, produce distinct clear sounds when struck with a hard object (a “hammer”). These sounds are unlike those occurring in nature. Consequently, they may have attracted the attention of toolmakers leading them to experiment with their production and acoustic properties.
When moving out of forested ecologies caves may have provided hominins with shelter and protection from predators – as they do for baboons where degree of inaccessibility to predators seems to be one of the factors involved in the choice of sleeping sites such as caves (Hamilton 1982). However, this may have been a double-edged sword as caves may have been dangerous for hominins as they may also serve as dens for predators (Treves and Palmqvist 2007: 359). Importantly, deposits in the Swartkrans caves contain fossilised remains of Australopiths, baboons as well as the extinct carnivorous cat Dinofelis. While there is some uncertainty in interpreting the remains, it has been suggested that early hominins may have been attacked while in the caves, a distinct possibility given that carnivores also use such caves as dens (Brain 1994; Treves and Palmqvist 2007: 363). Given increasing hominin occupation of caves as they ventured out of arboreal habitats, it is worth considering the degree to which sound production within such spaces may have effectively deterred predator attacks. Importantly for the evolution of sound production and auditory perception, there are resonant spaces inside cave structures that produce echoes (Fazenda et al. 2017). Additionally, reverberations in caves, particularly when occupied by a concentrated group of people, are thought to convert melodic intervals into harmonic intervals by prolonging the “tails” of preceding melodic tones (Nikolsky and Benítez-Burraco 2022). It is also worth speculating if early hominins may have explored the acoustic properties of caves, not only using their voices to produce sound, but also objects such as stone tools and perishable items that may not have survived in the archaeological record.
Given the above-mentioned evidence of chimpanzees swaying rhythmically to sound, synchronizing their movements to an auditory rhythm, as well as spontaneous whole-body entrainment between two peers, it does seem plausible that the hand morphology and tool making ability of Australopithecus would suggest increased capacity to produce sound and entrain to a felt pulse in ways more complex than that evident in chimpanzees. Significantly, entrainment seems to be associated with rhythmic knapping and collective manufacturing of stone tools (Zubrow and Blake 2006). Given Australopithecines were bipedal, which may have increased general rhythmic capabilities, as well as improving manipulative capacities due to the associated hand morphology, it seems possible that such forms of entrainment between numerous individuals may have been enhanced relative to other great apes. This perspective is consistent with the view that neural reorganization and expansion of motor regions associated with hand motor control may have emerged with the Australopithecines (Harmand et al. 2015).
It is important to reiterate the point that chimpanzees are limited in the extent to which they can develop the abilities outlined above due to the hand morphology associated with their locomotor adaptations. Additionally they would also lack the neurobiological substrates associated with both bipedal walking and associated forms of rhythmic perception – although the above examples of entrainment to a beat suggest chimpanzees may possess such abilities in an incipient form. However, we suggest that Australopiths may have evolved a more refined sense of rhythm due to being obligate bipeds – and this process of refinement would have continued in later species of hominin through autocatalytic feedback loops (Henneberg and Eckhardt 2022). Significantly, there is evidence in Australopithecus africanus of an external auditory meatus more conducive to the perception of high frequencies than that evident in chimpanzees, a derived hominin trait that is thought to facilitate short-range intragroup communication in open habitats (Quam et al. 2015). Combined with the increased ability to manufacture and use hammers and anvils, which may have produced loud rhythmically sequenced sound, there seems to be a plausible case for Australopithecus using synchronized sound production and body movement as an adaptation to non-arboreal ecologies with elevated predation risk.
It has been argued that our ancestors were vulnerable hominins living in open habitats with limited weaponry, and that they may have survived by increasing the range and diversity of their vocal calls. For example, ‘…lions prowling in the dark may have been more wary of approaching a noisy bunch of females and infants if unexpected pitch variations made it difficult to estimate group size and risk’ (Knight and Lewis 2017:437). Group rhythmic singing and dancing has also been called an effective intimidation tool that may have been a survival strategy of ancestral hominins when they moved out of forested habitats (Jordania 2020). Such synchronized vocalization and body movement may have been combined with hitting stones (hammers on anvils) resulting in vigorous ‘drumming sessions’ during scavenging confrontations with large African predators such as lions (Jordania 2014: 94).
As already noted many primate species use vocalisation in their attempt to deter predators. Some primate species may use alarm calls to deter predators that depend on surprise attacks – that is such calls communicate to the predator that they have been seen and that consequently it is unprofitable to continue the attack (Zuberbühler et al. 1999). They may also combine vocalisations with physical attack in mobbing behaviour, which involves two or more prey animals distracting or repelling a predator by making repeated advances usually while vocalizing and displaying in a conspicuous fashion (Treves and Palmqvist 2007: 368).
Chimpanzees have been observed uttering despaired calls while climbing up into a big tree (Boesch 1991: 228); effectively chasing leopards away using loud synchronized barking and branches to fight them off, with group level co-operation mediated by vocal calls being one of the most effective means of predator defense (Boesch 2009:22–23 and 52–53); responding to lions by climbing in to trees and eliciting alarm calls and whimpers (Tsukahara: 1993); as well as climbing high into trees where they remained uttering frequent loud vocalizations until potential predators left the area (Tutin et al. 1981: 139). In geladas, vocalisations may serve to alert other members of the group that a predator is nearby or to alert a leopard that it had been seen; additionally male geladas have been observed emitting loud barks and bluff‐charging to within three metres of a leopard while females and smaller juveniles sheltered in nearby trees and bushes (Lin et al. 2020: 11). Significantly, hunter-gatherer peoples use rhythmic clapping, drumming, chanting, and choral singing explicitly to keep wild animals away (Lewis 2009; Thin 1991).
In addition to vocalisation there seems to be a relationship between predation and social structure, with increased group cohesion reducing predation risk. For example, chimpanzee group level co-operation mediated by vocal calls may be one of the most effective means of predator defense in this species (Boesch 2009: 22–23 and 52–53). As Boesch notes, predation pressure in chimpanzees results in individuals of both sexes spending more time together, which means they are less likely to ‘be singled out in a leopard attack’ (Boesch 2009: 2). It is important to note that there also seems to be an ecological component in the relationship between social structure and predation. For example, savanna-living chimpanzees travel in large numbers when moving between patches of trees, which may be an anti-predator response (Tsukahara 1993), suggesting that as the number of trees decrease in a habitat group cohesion may become more important. Concurring with this observation, in baboons who adapted to open savanna or semidesert habitats where trees are rare as a source of protection from predators, resulted in the evolution of large aggressive males who can jointly drive predators away from the group (Kummer 1967: 154–155). Significantly, baboons have evolved extreme degrees of canine sexual dimorphism, which are in part related to the need for predator defense in what are essentially hostile savanna habitats with high predation risk (Plavcan and van Schaik 1992).
It has been argued that several million years ago, both hominins and the theropith ancestors of modern geladas transitioned from living in woodland-dominated habitats to more open‐country environments. The consequent reduction in the availability of refugia may have resulted in similar adaptations in both groups such as a fission‐fusion way of life and formation of multilevel societies (Lin et al. 2020). Significantly, geladas are thought to engage in vocal synchrony akin to human choral singing, both species using rhythm and melody to resolve emotional conflicts (Richman 1987). Therefore it seems possible that such socioecological adaptations may have been similar in geladas and Australopiths. In our model we hypothesize that elements of a prosocial adaptive suite, and possible cooperative breeding, were already in place in Ar. ramidus and that these adaptations were enhanced as Australopiths evolved social adaptations to cope with expansion into more diverse and challenging habitats.
Our analysis provides both fossil and palaeoecological support for the notion that elements of both music and language may have evolved from an evolutionary precursor form of “territorial chorus” (Brown 2017) – although, as we suggest below, such an adaptive complex may have been built upon preexisting mammalian neurochemistry. The “territorial chorus” thesis is based on the assumption that music and language evolved in the context of egalitarian social dynamics that promoted group-level communication, cohesion and co-operation (Brown 2007:16). In later periods of evolution, this primitive form of sound production may have bifurcated into sequentially structured language and harmonic musical forms, with such musical forms using isometric rhythms and pitch blends and language using words and propositional syntax (Brown 2001). In our model we propose that such a precursor may have already been evident in early hominins such as Ar. ramidus and that it was amplified through autocatalytic feedback loops (Henneberg and Eckhardt 2022) as Australopithecines moved into nonarboreal habitats and relied more and more upon group level sound production. This socio-behavioral shift may represent the very ancient building blocks of affect based sound communication in the hominin lineage – that is primordial hedonistic stimulation, which is believed to be a universal feature of music associated with affective bonding (Benítez-Burraco and Nikolsky 2023).
The concept of self-domestication has been proposed as a central component in human evolution (Hare 2017; Clarke and Henneberg 2015 and 2017). Based on analogies with domesticated animal breeds, this thesis seeks to explain certain observable trends in the hominin fossil record and features of modern human behavior and psychology; these include reduced cranial robusticity, shortened facial region, reduced levels of aggression, social tolerance, elevated levels of pro-sociality, reduced sexual dimorphism, increased disease risk and the retention of juvenile or paedomorphic features into adulthood (Hare 2017; Clark and Henneberg 2015; Leach 2003; Bednarik 2020). While the process has been postulated to explain the transition from more robust to more gracile and “feminized” morphology within the Homo genus (Cieri et al. 2014; Bednarik, Saniotis, and Henneberg 2022), it is also believed to have produced the more paedomorphic morphology and psychology of bonobos relative to chimpanzees (Hare, Wobber, and Wrangham 2012) as well as the paedomorphic skull architecture of Ar. ramidus, suggesting that the process of self-domestication began at the base of the hominin clade (Clark and Henneberg 2017, 2015).
Self-domestication is also believed to have been crucial to the evolution of music (Clark and Henneberg 2017; Benítez-Burraco and Nikolsky 2023) as well as being a precondition for the emergence of language (Thomas and Kirby 2018). This thesis is based on the assumption that language presupposes a system of social transmission and learning, and that such a system could have evolved through self-domestication. As the authors write, rather than ‘accounting for language structure itself, the key task for biological evolution lies in accounting for the foundational traits that make a process of structure-creating cultural evolution possible.’ Consequently, they argue that ‘the cultural evolution of language structure is rooted in an earlier process of self-domestication’ (Thomas and Kirby 2018:23).
Importantly, the skull morphology of Ar. ramidus shows greater similarity to infant chimpanzees than it does to adult chimpanzees (Fig. 1 and 2). These similarities include position of the foramen magnum, short relative length of the face and horizontal vocal tract in relation to the length of the skull, as well as the degree of cranial base flexion. Consequently, it has been argued that these paedomorphic features provide evidence for self-domestication at the base of the hominin clade (Clark and Henneberg 2017). It has also been argued that elevated levels of prosocial neurochemicals such as oxytocin may have evolved in Ar. ramidus (Lovejoy 2009; Clark and Henneberg 2017). It is important to note that elevated levels of oxytocin and serotonin are characteristic of species that have been domesticated by humans – further these neurochemicals seem to be part of the self-domestication complex of hominins (Hare 2017).
The important point to note here is that the changes in the Ar. ramidus skull that correlate with elevated levels of pro-sociality and evidence for self-domestication, are the same that are required to evolve skull morphology and a vocal tract necessary for vocal modulation – that is a centrally positioned foramen magnum (itself a product of erect bipedalism), loss of canine armory and reduced facial prognathism (Clark and Henneberg 2017). This observation provides detailed anatomical evidence for the contention that ‘the cultural evolution of language structure is rooted in an earlier process of self-domestication’ (Thomas and Kirby 2018: 23). As noted by Clark and Henneberg (2017), these deep interconnections between the anatomical basis of both vocal tract anatomy and social evolution evident in early hominins, have yet to be adequately explored by paleoanthropologists and scholars researching the origins of music and language. We hope this paper goes some way to rectifying this lacuna in the extant literature. In what follows we elaborate on this perspective in more detail, highlighting how neurochemical regulation and the process of self-domestication may have facilitated the growth of technology and musical ability throughout the course of hominin evolution.
It has been argued that the evolution of human mental capacities was not particularly dependent on changes in brain size and structure, but rather on alteration of its neurotransmitter and neurophysiological regulation involved in information processing and emotional states (Previc 2009; Saniotis et al. 2019; Henneberg and Saniotis 2016; Previc 1999; Saniotis and Henneberg 2012). However, current knowledge of neurobiological processes underlying cognitive abilities in ancestral hominins is still scant since neuro-biochemical effects do not fossilise (Saniotis and Henneberg 2011). That said, in the above discussion we have made some tentative suggestions in which we have sought to infer changes in neurochemical profiles that may have accompanied changes in skeletal anatomy – which do fossilize. This was based on comparison with other species and evidence of neurochemical regulation associated with the self-domestication syndrome.
In what follows we discuss the role of oxytocin, serotonin, and dopamine and how these neurochemicals may be related to the fossil and paleo-ecological evidence discussed above. Importantly, it has been argued that the process of enculturation was largely influenced by neuro-hormonal regulation, especially from the Paleolithic period onwards where sophisticated social behaviors, technology and art developed (Bednarik et al. 2022) and that the social transmission of linguistic structures was dependent on self-domestication (Thomas and Kirby 2018) which involves altered neurochemical regulation (Hare 2017).
Oxytocin is a deeply conserved neurochemical primarily associated with female mammalian nurturant behavior (Panksepp 2004). Importantly, in both bonobos and chimpanzees the oxytocin system is involved in group cohesion – but it functions in species specific ways. For example, in chimpanzees the oxytocin system seems to be associated with in-group bonding particularly in the context of intergroup aggression, while in bonobos it seems to facilitate both in-group cohesion as well as affiliative behaviour between groups (Brooks et al. 2022). Given chimpanzee in-group cohesion is believed to be related to predation pressure and intergroup conflict (Boesch 2009), it seems the oxytocin system can facilitate sociality in the face of external threat or competition. However, it is unlikely that these are the reasons for the oxytocin system evolving in chimpanzees – that is it is more likely the oxytocin system, originally evolving in the context of female mammalian nurturant behavior, was exapted in adulthood group bonding. That the oxytocin system can be exapted in species specific ways, is evidenced by the fact that in bonobos it is not associated with intergroup conflict but forms the physiological basis for increased motivation to cooperate as well as intergroup affiliation (Moscovice et al. 2019).
In humans oxytocin is involved in sexual bonding (Light, Grewen, and Amico 2005; Panksepp and Biven 2012: 241) parental psychology (Gordon et al. 2010) as well as generalised co-operation (Rilling et al. 2012). It is also involved in the social dimensions of music, such as trust and cooperation within groups of culturally compatible but not necessarily genetically related individuals – aspects of sociality that are believed to stimulate reward and motivation due to music’s impact on the limbic system (Harvey 2020). Importantly, dance has been shown to induce pleasurable arousal and positive prosocial mood via the release of endorphins and neurohormones such as oxytocin (Laland, Wilkins, and Clayton 2016). The oxytocin system may also be associated with a form of primordial hedonistic stimulation, a putatively universal feature of music associated with affective bonding that underpins diverse cultural forms of music expression (Benítez-Burraco and Nikolsky 2023).
While the oxytocin system, and its role in the evolution of early hominin musical behavior, may have been important for group cohesion in the face of external threat or intergroup conflict – as it is in chimpanzees – it may not have originally evolved for such purposes. For example, it has been argued that the oxytocin system and the coevolved ritualized facial, bodily, and vocal signals of affiliative intent by ancestral mother–infant pairs, formed the original adaptation that was then exapted in music, dance, group ritual and other social bonding behaviours (Dissanayake 2021).
This perspective has been developed in the context of the Australopithecine demographic expansion, suggesting that “motherese” or infant-mother vocalisations were able to establish a form of nontactile contact comfort, which then provided the neurobiological basis of adulthood music and social bonding (Falk 2004). This theory is related to the vertical vector of bipedalism, which makes it difficult for hominin infants to ride on their mother’s back in the manner of quadrupedal apes such as chimpanzees. Additionally, a putative decrease in infant grasping abilities, and specifically the lack of a grasping big toe associated with bipedalism, would have required mothers to place their infants on the ground while they foraged, with reciprocal vocalisation maintaining nontactile contact (Falk 2004).
While the notion of such infant-directed speech has been found to exist in various forms cross-culturally suggesting an evolved universal communicative system (Hilton et al. 2022), the motherese thesis has been called into question due to a lack of anthropological support for the theory (Rosenberg, Golinkoff and Zosh 2004). While accepting aspects of the motherese thesis, and the possibility that mother infant vocalisations may have formed the neurobiological substrates of prosocial melodic vocalisation, we see this form of social bonding in a broader social context, and that infant vocalisations may also serve to elicit responses from not just the mother but also alloparents – a position that does seem to find support from cross cultural data (Hrdy 2009: 123). In this sense vocally mediated bonding between infants, the mother and other members of a cooperatively breeding social unit, may have been exapted in adult social bonding, providing the neurobiological foundation and ontogenetic precursors for such socioemotional traits – as has been claimed for cooperation, empathy and altruism more generally (Preston 2013). Given oxytocin functions in species specific ways in bonobos and chimpanzees, it likely would have also done so in early hominins – and as is the case with modern humans it may have subserved early forms of coordinated movement and sound production.
Various authors have suggested that serotonergic regulation became increasingly employed in the hominin clade where it functioned in impulse control and delayed gratification (Raghanti et al. 2008; Azmitia 1999; Saniotis et al. 2021; Soubrié 1986). Furthermore, it has also been found that increasing serotonin levels in the striatal areas of the brain further reinforced limbic inhibition which was crucial in the development of tool production, language and affiliative behaviors (Raghanti et al. 2018). It has also been suggested that the serotonin receptor 5-HT2AR may have had a significant role in human evolution by improving neuroplasticity and adaptive behaviors in adverse environments (Ettrup et al. 2014). This has obvious implications for hominin expansion into nonarboreal habitats with increased predation risk.
Significantly, serotonin and BDNF (brain derived neurotropic factor) work to reinforce each other. For instance, BDNF enhances serotonergic expression of raphe neurons, as well as upregulating serotonergic uptake and modifying serotonergic neuron firing rates (Goggi et al. 2002; Martinowich and Lu 2008; Zhou, Sari, and Zhang 2000). It has also been argued that BDNF underwent positive selection due to increasing physical activity levels (i.e. persistent hunting) from H. ergaster onwards, which produced more BDNF which in turn enhanced its synergistic neurotrophic and cognitive roles with the serotonergic system (Saniotis and Henneberg 2013). Further, alteration in BDNF expression may have been exapted in early hominin social activities which entrained affective states via rhythmic motor sequences – for example those involved in dance (Brown, Martinez, and Parsons 2006). Importantly, dance has been shown to increase neurotrophins such as BDNF which assist in neuroplasticity and cognitive function (Brown, Martinez, and Parsons 2006) while music, dance and ritual are believed to result in forms of prosocial synchronization and merging between self and other facilitated by endorphin release (Tarr, Launay and Dunbar 2014). While it is unclear when these neurochemicals were upregulated in the hominin lineage, we suggest that such a process may have begun with early hominins such as Ar. ramidus, and particularly the Australopithecines when coordinated movement, sound production and vocalisation increased social cohesion and predator defence in nonarboreal habitats.
It is important to note the lifestyle and dietary changes that occurred as hominins ventured into nonarboreal habitats and developed a broader diet. It has been suggested that the climatic changes occurring over the last several million years in sub-Saharan Africa contributed to increased meat consumption and elevated thyroid and dopamine production as hominins expanded their locomotor range, engaged in chase hunting, and adapted to ecologies inducing increased thermoregulatory stress (Previc 2009, 1999). Additionally, the consequent increasing dietary levels of the omega-3 DHA in ancestral hominins probably increased thyroxine (T4) which is involved in creativity, language fluency and memory (Previc 2002). Thyroxine is implicated in converting tyrosine to the dopamine precursor L-Dopa. Significantly, it has been shown that T4 levels in humans are approximately 30% higher than in chimpanzees (Previc 2002).
Dopamine is a significant neurotransmitter which is involved in planned movement, neuromodulation, spatial memory, motivational behaviour and cognitive function (Klein et al. 2019; Berridge and Kringelbach 2008; Salamone and Correa 2012). The medial caudate nucleus which forms the striatum with the putamen in basal nuclei has increased dopaminergic activity compared to non-human primates, supporting more flexible cognitive abilities and behaviours (Raghanti et al. 2016). Importantly, dopamine is believed to be an important factor in sequential learning and planning of motor tasks (Badgaiyan, Fischman, and Alpert 2007). Additionally, the motor system is thought to play a central role in musical and rhythmic perception, suggesting that motor planning is not only involved in movement but is also recruited for music perception even in the absence of actual physical movement (Gordon, Cobb, and Balasubramanian 2018). We suggest that the regulation of oxytocin, serotonin and dopamine would have been under significant selective pressure as hominins adapted to changed ecological conditions, resulting in enhanced prosocial behaviors, motor control and synchronized sound production and bodily movements.
The model outlined in this article focusing on anatomy, social structure and neurochemical regulation may prove useful given the discovery of small brained hominins such as Homo naledi. For example, Homo naledi possesses many human-like anatomical traits associated with the hand, foot, lower limb, dentition and cranium – yet significantly it has a brain size equal to that of australopiths (Berger et al. 2017). What this suggests is that posture, hand morphology, altered neural architecture and associated neurochemical regulation may give rise to what were once considered uniquely human social behaviors – behaviors that, based on the Homo naledi evidence, do not seem to require large brain size. For example, recent excavations have led to claims that Homo naledi seems to possess cultural traits characteristic of modern humans, including engraving, fire and using forms of collaborative planning and coordination to bury their dead in caves (Fuentes et al. 2023). Given the occupation of caves by this species, is it possible that Homo naledi took pleasure in, and experimented with, the resonant spaces and reverberatory potential of caves? To our knowledge there is no evidence as yet that they did so – however absence of evidence is not evidence of absence. While the findings of the Homo naledi excavations are promising, and potentially revolutionary for our understating of hominin evolution, more research is required to establish the veracity of the claims mentioned above; for a critical analysis of these claims and possible avenues for further research see reviewers’ comments in Berger et al. (2023). One possible avenue of future research may be to ascertain any previously unnoticed evidence of musical behaviors among Homo naledi artefacts.
Our model may also help illuminate the neurobiological substrates of social cognition among the Dmanisi hominins, which have cranial capacity ranging from 545 to 760 ml (Lordkipanidze 2017, p. 49). Importantly, the lower limit of the Dmanisi fossils of 545ml is only slightly larger than the upper limit of chimpanzees, which is 500ml (Tobias 1971). It is unlikely that such a small difference in brain size can account for the differences in ecological niche, subsistence patterns and socio-behavioral adaptations between these two species.
Significantly, the Dmanisi hominins are believed to have used an Oldowan Mode 1 tool kit, to have been cooperative hunters who had access to large game, who had a diet broader than H. habilis (Lordkipanidze et al. 2013; Pontzer, Antón, and Lordkipanidze 2014) and who were highly social in terms of care for conspecifics (Lordkipanidze et al. 2005). Given that geographic dispersal is believed to be dependent on high levels of sociality, combined with the small body and brain size of the Dmanisi fossils, it has been suggested that the earliest hominin presence in Eurasia predated increases in body size and brain size (Lordkipanidze et al. 2013). Given the small brain size of these hominins, combined with evidence of many aspects of the human adaptive suite, it seems reasonable to propose that their psychosocial adaptations result from altered brain architecture and neurochemical regulation associated with hominin bipedalism. Significantly, there is evidence of antemortem damage to the fossils that has been attributed to predation (Margvelashvili 2022; Lordkipanidze et al. 2023). It is interesting to speculate the possible forms of sound production employed by these small brained hominins – given they could make stone tools and given evidence of possible predation would they have used coordinated sound production to deter predators? That is given their ability to engage in complex forms of sequential motor control in the making of weapons and tools, we also suggest that these hominins may have employed these abilities in forms of synchronized sound production and bodily movement – to either enhance group cohesion or as a form of predator defense.
In this article we have argued that the sequential processing and neurochemical regulation resulting from erect bipedalism may have evolved through autocatalytic feedback loops that can be traced back to early hominins such as Ar. ramidus and the Australopithecines (Henneberg and Eckhardt 2022). We also suggested that increased capacity for such processing and enhanced motor control, in the context of a prosocial egalitarian social system, is essential for human technological evolution and the structural properties of human communicative capacities. As opposed to seeing modern linguistic and behavioral capacities evolving with the emergence of larger brained and more gracile members of the Homo genus (Diniz-Filho et al. 2019; Benítez-Burraco and Kempe 2018; Neubauer, Hublin, and Gunz 2018) we suggest that many of the adaptations associated with behavioral modernity were already in place among Homo erectus (Bednarik 2013; Webb 2006; Sterelny 2012; Bednarik 2015) and that they originally evolved through a social niche involving self-domestication processes, cooperative breeding and intergenerational knowledge transfer that may reach back to Ar. ramidus and the Australopiths (Clark and Henneberg 2021a; Clark and Henneberg 2021b). In the following we explore this perspective in greater detail as a means of interpretating the archeological evidence for music – from ancient lithophones to purported flutes found by archaeologists from the Aurignacian, a technocomplex of hominins transitional between robust and gracile Homo sapiens, i.e., Neanderthaloid humans (Bednarik 2020).
It has been argued that self-domestication led to increased skull gracility and globularity within the Homo genus, which was accompanied by changes in neural architecture that gave rise to modern forms of cognition and language use within anatomically modern humans (Benítez-Burraco and Kempe 2018). However, this position has been questioned based on evidence that other factors such as mastication may contribute to differences between robust and gracile members of the Homo genus – and that consequently robust varieties going back as far as 600k may have been behaviourally modern (Clark and Henneberg 2021). If this position turns out to have any merit then the question arises: to what degree did music form part of behavioural modernity in earlier varieties of the Homo genus?
One of the problems in establishing the evolutionary origins of music is most types of musical instrument would have been made of perishable materials usually lost from the archaeological record due to taphonomic processes (Bednarik 1994). This also means that the absence of such artifacts does not necessarily mean that earlier hominins lacked the socio-cognitive ability to produce and use them. As opposed to inferring the emergence of musical abilities by reference to archaeological finds of musical instruments, we interpret such evidence as some of the most recent examples of hominin sequential processing, rhythmic perception and musical abilities that occurred as result of architectural and neurochemical alterations of the hominin brain.
Significantly, in palaeoart, we have a number of finds that are attributable to robust rather than gracile hominins (Bednarik 2017), and there is a tantalisingly small number of apparent musical instruments from Middle Palaeolithic contexts. About thirty presumed flutes or pipes have been reported from Upper Palaeolithic settings, but as mentioned, they need not necessarily be attributed to gracile H. sapiens. The best-known examples are those from the Aurignacian of Hohle Fels, Vogelherd and Geiβenklösterle in Germany (Hahn and Münzel 1995; Conard, Malina, and Münzel 2009), Spy in Belgium (Otte 1979), and Isturitz, Abri Blanchard and Mas d’Azil in France (Passemard 1944; Harrold 1988).
As already noted, caves are an important competent in hominin evolution going back to at least Homo naledi if not the Australopithecines. Caves may have provided shelter and protection from predators in the absence of arboreal forms of refuge as well as being places of symbolic or ritual behaviour (Jaubert et al. 2016). Importantly, many of the paintings of the Upper Paleolithic are situated in the most resonant areas of the caves, which includes stalactites which are reported to have been used as natural tone producing ‘lithophones’ (Morley 2013: 115–117). Significantly, it has been argued that the acoustic properties of such spaces amplify pitch value directing the attention of singers to fundamental frequencies and harmonicity (Benítez-Burraco and Nikolsky 2023). Additionally, the acoustic properties of caves have led to the suggestion that early forms of music and visual art may have developed as part of cave based ritual practices (Morley 2013).
Some researchers have argued that Neanderthals exhibit evidence of cave based behaviours traditionally associated with Homo sapiens. For example, the Middle Palaeolithic Bruniquel Cave deposits in France contain evidence of anthropogenic geometric structures made of stalagmites, suggesting forms of social organization among Neanderthals more complex than previously thought (Jaubert et al. 2016). However, it is unclear how ancient forms of ritual and associated musical behaviors are. We suggest it is likely they emerged prior to the evolution of anatomically modern humans, having developed among robust hominins such as Neanderthals. It may even be the case that very early versions of ritual behavior, at least in incipient form, were present among small brained hominins as suggested by the Homo naledi finds. It will be interesting to see how future research illuminates this issue further.
Importantly, predation on hominins appears to have been common during the Pleistocene among early anatomically modern humans as well as Neanderthals (Camarós et al. 2016). For example Neanderthal fossils from The Cova Negra in Spain have cranial punctures similar to those evident in the Australopithecus cranial fragment SK-54 from Swartkrans in South Africa, which are believed to have resulted from a leopard attack (Camarós et al. 2016). It is unclear if cave occupation during this period was a response to predation pressure or other factors. What seems to be clear is caves were a place conducive to the production and amplification of sound, that early members of the Homo genus ocupied them, and that they most likely did so for shelter and possible cultural reasons. Predation may have been one factor engendering this practice although it seems diffcult at present to ascertain its realtive role with any certainty.
There have been occasional reports of flute-like objects from Middle Palaeolithic contexts, of which one example deserves closer attention (Fig. 3). It is the Mousterian bone flute from Divje babe I, Slovenia, from layer 8, the lowest of five Mousterian strata (Turk 1997; Turk, Dirjec, and Kavur 1995). Since it was first reported, it has led to intensive debates (d’Errico et al. 2003; Chase and Nowell 1998; d’Errico and Stringer 2011; d’Errico and Villa 1997). The tubular fragment of a juvenile cave bear femur bearing a series of holes is dated to about 50 ka. Advocates of the replacement of replacement of robust members of the Homo genus by more gracile forms, argue that the four regularly spaced holes result from carnivore activity, yet they lack indications of compression or crushing and any counter-traces on the underside. Moreover, experimentation has demonstrated that the object has a two-and-a-half-octave compass that extends to over three octaves by over-blowing, and perfectly melodious tunes can be played on it (Turk et al. 2018). It would be readily accepted as a flute if it were from an Aurignacian context because replacement scholars attribute that musical tradition to gracile H. sapiens.
Fig. 3. Presumed flute of the Mousterian of Divje babe I, Slovenia, made of a cave bear bone
Flutes or pipes are not the only musical instruments described from Palaeolithic contexts. Skiffles, rasps or scrapers have been reported from several Upper Palaeolithic occupation layers (Geiringer 1982: 13–14; Kuhn and Stiner 1998; Huyge 1991; Maringer 1982; Vincent 1988; Dauvois 1989, 1999). One such possible scraped idiophone is attributable to the Middle Palaeolithic. It is the fragment of a mammoth long bone (probably a femur or tibia) found with a cold fauna and Mousterian flint implements at Schulen, Belgium (Huyge 1990). The oblique fracture to create a pointed end was achieved by a cut scored with a stone tool, along which the bone was then snapped off. The deeply cut subparallel grooves extending from the point downwards (Fig. 4) have been so intensively worn by transverse rubbing, especially near the point, that their original number is difficult to establish. There seem to have been twelve grooves initially, and their separating ribs, as well as the edge’s underside, are covered by an intense tribological gloss featuring micro-striations parallel to the tool’s edge. The gloss is absent in the groove floors, which have retained the longitudinal striae of the lithic tools used to create them.
Fig. 4. Presumed skiffle on a mammoth bone fragment from Schulen, Belgium
Free aerophones, usually known as bullroarers, have also been attributed to the Palaeolithic, as have osseophones (struck bones), albeit with less persuasive examples. The evidence is much clearer for another form of idiophone, the lithophones. These are rocks struck to vibrate, producing clear sounds audible over great distances (Boivin et al. 2007; Boivin 2004). Lithophones can be identified and may provide the earliest evidence available of music-making – although we suggest this ability may in fact be an extension of the hominin sound producing capability that can be traced back to Lomekwi artefacts. The quality and tone of the sound they yield depend on the shape and material properties of the stone and the amount of contact it has with other rocks: minimal contact facilitates more effective vibration. Therefore, stalactites in limestone caves are acoustically suitable candidates. Several cave sites have been proposed to have furnished such evidence in the form of marked or struck speleothem formations (Glory 1964, 1965; Vaultier, Santos, and Glory 1965), such as the ‘organ sanctuary’ in Nerja Cave, Spain (Dams 1984, 1985). However, these are Upper Palaeolithic, although the Escoural Cave examples could conceivably be Middle Palaeolithic as Upper Palaeolithic occupation evidence is lacking at that site.
Most known lithophones (Querejazu Lewis and Bednarik 2010) or ‘rock gongs’ are large slabs of rock featuring cupules, a type of cup-shaped indentation that is the most common petroglyph on the planet (Bednarik 2008). These may occur singly on the lithophone’s spot most conducive to emitting good sound, but different parts of a lithophone may yield different notes (Bednarik 2010a). Therefore, most specimens bear several cupules, even hundreds (Fig. 5). Cupules were made by percussion and can occur on any rock type, and most of them are not found on rocks suitable as lithophones. They have been made from the Lower Palaeolithic to the 20th century (Fig. 6). Replication studies have determined that those made on the hardest rocks, such as granite, quartzite and in rare cases, even massive quartz, can require up to hundreds of thousands of blows with stone hammers (Kumar and Krishna 2014). Most of the earliest known cupules occur on rocks that may not be good lithophones, such as those at Daraki-Chattan (India), Nchwaneng and Potholes Hoek (both South Africa) or Sai Island (Sudan) (Bednarik 2017: 43–44, 115–118). A notable exception is the upright quartzite slab in Auditorium Cave (India; op. cit. Fig. 38), located in a space of distinctive acoustic properties. The cupules of these five sites have been attributed to the Lower Palaeolithic, although dating these phenomena remains notoriously difficult (Bednarik 2010b).

Fig. 5. Granite lithophone covered by cupules; Serra do Papagaio III, Santana do Matos, Rio Grande do Norte, Brazil

Fig. 6. Some of the 550 cupules on the walls of the quartzite cave Daraki-Chattan, Bhanpura, central India, 28 of which have been excavated in Lower Palaeolithic strata
Of particular interest here are the sensory effects of the rhythmic sound of cupule production sessions, combined with the tremendous physical exertion demonstrated by the replication experiments that revealed the involvement of immense ‘commitment, stamina and patience’ (Kumar and Krishna 2014). Cupules on tough rock, especially those on lithophones, could have been either produced by countless short sittings over many generations; or they may be the result of many monotonous sessions lasting hours at a time, introducing a trance-like state in the operative. The timing of striking the rock favours intervals reflecting the rebound characteristics of the stone hammers, i.e., strikes are very precisely spaced in time. The kinetic mechanics of the process manifest the tribological properties of the elements involved, establishing a specific rhythm, and it can seem to the operator that the rock determines the rate of striking. This establishment and governing of rhythm over a lengthy period, combined with distinctive pitches of sound produced, is a prime candidate for embedding the rudiments of music production. Although it does not fix the timing of the advent of music production any more than the archaeological data listed above, any form of natural patterning would have helped hone hominin cognition. Further, such evidence for cognitive honing may be an extension of processes that can be traced back to the earliest stone tool technologies.
In conclusion, our analysis offers a model of human music origins and dance grounded in the palaeoecological contexts of early hominin evolution. We propose that changes in skull architecture, locomotion, hand morphology, neurobiology and ecology, may have favored the uses of coordinated sound production and movement as part of a more generalized adaptive suite. We further argue that this very primitive form of movement and sound production provided the phylogenetic building blocks that were consequently amplified though autocatalytic feedback loops during subsequent periods of hominin evolution.
It is also a model that opens up potentially new avenues of research. For example, some stone tools have been shown to have traces of use as sound producing objects. The veracity of our model may be able to be tested by re-analyzing Pleistocene artefact assemblages for any hints of their use as “sound tools”. As Blake and Cross argue, the realisation of the potential – and often overlooked – sound producing properties of stone tools may necessitate the ‘seemingly monumental task of re-analysing or re-considering excavated lithic collections’ a research project that ‘should target sites where other forms of musical or quasi-musical behaviours have survived’ (Blake and Cross 2008: 17). The presence of such evidence among early hominin archaeological deposits could be used to test our model. If such evidence was forthcoming it would push back music origins into much earlier periods of paleohistory than previously thought. We predict such an archaic origin for musical abilities based on the unique anatomical configuration, and associated neurobiology, of hominin bipedalism, canine anatomy and hand morphology. The challenge for future researchers is to discover clues for such an ancient origin despite the taphonomic processes that make finding such evidence in older archaeological layers less likely.
Conflict of interests
Authors declare no conflict of interests.
Authors’ contributions
GC was the primary author of the paper. All authors were involved in the discussion and conception of the hypothesis. AS and ML contributed material on neurohormonal regulation and RB on archaeology. MH contributed material on palaeoanthropology and developed the overarching conceptual orientation.
Almécija S, Moyà-Solà S, Alba D. 2010. Early Origin for Human-Like Precision Grasping: A Comparative Study of Pollical Distal Phalanges in Fossil Hominins. PLOS ONE 5 (7):e11727. https://doi.org/10.1371/journal.pone.0011727
Arcadi A, Robert D, Boesch C. 1998. Buttress drumming by wild chimpanzees: Temporal patterning, phrase integration into loud calls, and preliminary evidence for individual distinctiveness. Primates 39 (4):505–518. https://doi.org/10.1007/BF02557572
Azmitia EC. 1999. Serotonin neurons, neuroplasticity, and homeostasis of neural tissue. Neuropsychopharmacology 21 (2 Suppl):33s–45s. https://doi.org/10.1016/S0893-133X(99)00022-6
Badgaiyan RD, Fischman A, Alpert NM. 2007. Striatal dopamine release in sequential learning. Neuroimage 38(3):549–56. https://doi.org/10.1016/j.neuroimage.2007.07.052
Baldwin PJ, Pí JS, McGrew WC, Tutin CEG. 1981. Comparisons of nests made by different populations of chimpanzees (Pan troglodytes). Primates 22(4):474–486. https://doi.org/10.1007/BF02381239
Bednarik RG. 1994. A taphonomy of palaeoart. Antiquity 68:258.
Bednarik RG. 2008. Cupules. Rock Art Research (AURA) 25(1):61–100.
Bednarik RG. 2010a. About lithophones. In: RG Bednarik, editor. Mysterious cup marks: proceedings of the First International Cupule Conference, Oxford: Archaeopress.
Bednarik RG. 2010b. Estimating the age of cupules. Paper read at Mysterious Cup Marks. Proceedings of the First International Cupule Conference. BAR International Series.
Bednarik RG. 2013. Creating the Human Past: An Epistemology of Pleistocene Archaeology: Archaeopress.
Bednarik RG. 2015. The First Mariners. Bentham Science Publishers.
Bednarik RG. 2017. Palaeoart of the Ice Age. Cambridge Scholars Publishing.
Bednarik RG. 2020. The Domestication of Humans. Taylor & Francis.
Bednarik RG, Saniotis A, Henneberg M. 2022. Auto-domestication hypothesis and the rise in mental disorders in modern humans. Medical Hypotheses 164:110874. https://doi.org/10.1016/j.mehy.2022.110874
Begun DR. 2018. The Real Planet of the Apes: A New Story of Human Origins: Princeton University Press.
Begun DR, Ward C, Rose M. 1997. Function, Phylogeny and Fossils: Miocene Hominoid Evolution and Adaptations. Springer US.
Beliveau V, Ganz M, Feng L, Ozenne B, Højgaard L, Fisher P, Svarer C, Greve DN, Knudsen GM. 2017. A High-Resolution In Vivo Atlas of the Human Brain’s Serotonin System. J Neurosci 37(1):120–128. https://doi.org/10.1523/JNEUROSCI.2830-16.2016
Bengtsson SL, Ullén F, Ehrsson H, Hashimoto T, Kito T, Naito E, et al. 2009. Listening to rhythms activates motor and premotor cortices. Cortex 45(1)62–71. https://doi.org/10.1016/j.cortex.2008.07.002
Benítez-Burraco A, Kempe V. 2018. The Emergence of Modern Languages: Has Human Self-Domestication Optimized Language Transmission? Front Psychol 9(551). https://doi.org/10.3389/fpsyg.2018.00551
Benítez-Burraco A, Nikolsky A. 2023. The (Co)Evolution of Language and Music Under Human Self-Domestication. Human Nature 34(2)229–275. https://doi.org/10.1007/s12110-023-09447-1
Berger LR, Hawks J, Dirks P, Elliott M, Roberts E. 2017. Homo naledi and Pleistocene hominin evolution in subequatorial Africa eLife 6:e24234 https://doi.org/10.7554/eLife.24234
Berger LR, Makhubela T, Molopyane K, Krüger A, Randolph-Quinney P, et. al. 2023. Evidence for deliberate burial of the dead by Homo naledi. eLife 12:RP89106 https://doi.org/10.7554/eLife.89106.1
Berger LR, Hawks J, Fuentes A, van Rooyen D, Tsikoane M, et. al. 2023. 241,000 to 335,000 Years Old Rock Engravings Made by Homo naledi in the Rising Star Cave system, South Africa. eLife 12:RP89102 https://doi.org/10.7554/eLife.89102.1
Berridge KC, Kringelbach ML. 2008. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 199(3):457–480.
Blake E, Cross I. 2008. Flint Tools as Portable Sound-Producing Objects in the Upper Palaeolithic Context: An Experimental Study.
Blake E, Cross I. 2015. The Acoustic and Auditory Contexts of Human Behavior. Curr Anthropol 56(1)81–103. https://doi.org/10.1086/679445
Boesch C. 1991. The Effects of Leopard Predation On Grouping Patterns in Forest Chimpanzees. Behaviour 117:220–241.
Boesch C. 2009. The Real Chimpanzee: Sex Strategies in the Forest: Cambridge University Press.
Böhme MN, Spassov J, Fuss A, Tröscher AS, Deane J, Prieto U, Kirscher U, Lechner T, Begun DR. 2019. A new Miocene ape and locomotion in the ancestor of great apes and humans. Nature 575(7783):489–493. https://doi.org/10.1038/s41586-019-1731-0
Boivin N. 2004. Rock art and rock music: Petroglyphs of the south Indian Neolithic. Antiquity 78:38–53. https://doi.org/10.1017/S0003598X00092917
Boivin N, Brumm A, Lewis H, Robinson D, Korisettar R. 2007. Sensual, material, and technological understanding: exploring prehistoric soundscapes in south India. JRAI 13:267–294. https://doi.org/10.1111/j.1467-9655.2007.00428.x
Brain CK. 1983. The Hunters Or the Hunted?: An Introduction to African Cave Taphonomy. University of Chicago Press.
Brain CK. 1994. The Swartkrans palaeontological research project in perspective: results and conclusions. S Afr J Sci 90(4)220–223. https://doi.org/10.10520/AJA00382353_5380
Brooks J, Kano F, Kawaguchi Y, Yamamoto S. 2022. Oxytocin promotes species-relevant outgroup attention in bonobos and chimpanzees. Horm Behav 143:105182. https://doi.org/10.1016/j.yhbeh.2022.105182
Brown S, Martinez M, Parsons LM. 2006. The neural basis of human dance. Cereb Cortex 16(8):1157–1167.
Brown S. 1999. The “Musilanguage” Model of Music Evolution. In: S Brown, B Merker, C Wallin, editors. The Origins of Music. The MIT Press.
Brown S. 2001. Are Music and Language Homologues? Ann N Y Acad Sci 930. https://doi.org/10.1111/j.1749-6632.2001.tb05745.x
Brown S. 2007. Contagious heterophony: A new theory about the origins of music. Music Sci 11(1):3–26. https://doi.org/10.1177/1029864907011001
Brown S. 2017. A Joint Prosodic Origin of Language and Music. Front Psychol 8:1894. https://doi.org/10.3389/fpsyg.2017.01894
Brown s. 2022. Group dancing as the evolutionary origin of rhythmic entrainment in humans, New Ideas in Psychology, Volume 64:1–12
Burkart JM, Fehr E, Efferson C, van Schaik CP. 2007. Other-regarding preferences in a non-human primate: common marmosets provision foodaltruistically. Proc Natl Acad Sci U. S. A. 104:19762–19766. https://doi.org/10.1073/pnas.0710310104
Camarós E, Cueto M, Lorenzo C, Villaverde V, Rivals F. 2016. Large carnivore attacks on hominins during the Pleistocene: a forensic approach with a Neanderthal example. Archaeol Anthropol Sci 8(3):635–646. https://doi.org/10.1007/s12520-015-0248-1
Cerling TE, Levin NE, Quade J, Wynn JG, Fox DL, Kingston JD, et al. 2010. Comment on the Paleoenvironment of Ardipithecus ramidus. Science 328(5982):1105–1105. https://doi.org/10.1126/science.1185274
Chanda ML, DJ Levitin. 2013. The neurochemistry of music. Trends Cogn Sci 17(4):179–193. https://doi.org/10.1016/j.tics.2013.02.007
Chaney ME, Ruiz CA, Meindl RS, Lovejoy CO. 2022. The foot of the human–chimpanzee last common ancestor was not African ape-like: A response to Prang (2019). Journal of Human Evolution 164:102940. https://doi.org/10.1016/j.jhevol.2020.102940
Chase PG, Nowell A. 1998. Taphonomy of a suggested Middle Paleolithic bone flute from Slovenia. Curr Anthropol 39(4):549–553. https://doi.org/10.1086/204771
Cheng L, Samuni L, Lucchesi S, Deschner T, Surbeck M. 2022. Love thy neighbour: behavioural and endocrine correlates of male strategies during intergroup encounters in bonobos. Anim Behav 187:319–330. https://doi.org/10.1016/j.anbehav.2022.02.014
Choi JY, Takahashi DY, Ghazanfar AA. 2015. Cooperative vocal control in marmoset monkeys via vocal feedback. J Neurophysiol 114:274–283. https://doi.org/10.1152/jn.00228.2015
Cieri RL, Churchill S, Franciscus R, Tan J, Hare B. 2014. Craniofacial Feminization, Social Tolerance, and the Origins of Behavioral Modernity. Curr Anthropol 55(4):419–443. https://doi.org/10.1086/677209
Clark G, Henneberg M. 2015. The life history of Ardipithecus ramidus: A heterochronic model of sexual and social maturation. Anthropol Rev 78(2). https://doi.org/10.1515/anre-2015-0009
Clark G, Henneberg M. 2017. Ardipithecus ramidus and the evolution of language and singing: An early origin for hominin vocal capability. HOMO 68(2):101–121. https://doi.org/10.1016/j.jchb.2017.03.001
Clark G, Henneberg M. 2021. Interpopulational variation in human brain size: implications for hominin cognitive phylogeny. Anthropol Rev 84(4):405–429. https://doi.org/10.2478/anre-2021-0029
Clark G, Henneberg M. 2021. Cognitive and behavioral modernity in Homo erectus: skull globularity and hominin brain evolution. Anthropol Rev 84:467–485. https://doi.org/10.2478/anre-2021-0030
Conard NJ, Malina M, Münzel S. 2009. New flutes document the earliest musical tradition in southwestern Germany. Nature 460(7256):737–740. https://doi.org/10.1038/nature08169
d’Errico F, Henshilwood C, Lawson G, Vanhaeren M, Tillier A, Soressi M, Bresson F, Maureille B, Nowell A, Lakarra J, Backwell L, Julien M. 2003. Archaeological evidence for the emergence of language, symbolism, and music–an alternative multidisciplinary perspective. J World Prehist 17(1):1–70. https://doi.org/10.1023/A:1023980201043
d’Errico F, Stringer C. 2011. Evolution, revolution or saltation scenario for the emergence of modern cultures? Philos Trans R Soc Lond B Biol Sci 366(1567):1060–1069. https://doi.org/10.1098/rstb.2010.0340
d’Errico F, Villa P. 1997. Holes and grooves: the contribution of microscopy and taphonomy to the problem of art origins. J Hum Evol 33(1):1–31. https://doi.org/10.1006/jhev.1997.0141
Dams L. 1984. Preliminary findings at the ‘organ sanctuary’ in the cave of Nerja, Malaga, Spain. Oxf J Archaeol 3(1):1–14. https://doi.org/10.1111/j.1468-0092.1984.tb00112.x
Dams L. 1985. Palaeolithic lithophones: descriptions and comparisons. Oxf J Archaeol 4(1):31–46. https://doi.org/10.1111/j.1468-0092.1985.tb00229.x
Darwin C. 2004. The Descent of Man, and Selection in Relation to Sex. Penguin.
Dauvois M. 1989. Son et musique paléolithiques in La musique dans l’Antiquité. Dossiers d’Archéologie (Les) (142):2–11.
Dauvois M. 1999. Mesures acoustiques et témoins sonores osseux paléolithiques. Paper read at Préhistoire d’os (recueil d’études sur l’industrie osseuse préhistorique offert à Henriette Camps-Fabrer).
de Bonis L, Koufos GD, Andrews P. 2001. Hominoid Evolution and Climatic Change in Europe: Volume 2: Phylogeny of the Neogene Hominoid Primates of Eurasia. Cambridge University Press.
Diniz-Filho J, Jardim L, Mondanaro A, Raia P. 2019. Multiple Components of Phylogenetic Non-stationarity in the Evolution of Brain Size in Fossil Hominins. Evol Biol 46(1):47–59. https://doi.org/10.1007/s11692-019-09471-z
Dissanayake E. 2021. Ancestral human mother–infant interaction was an adaptation that gave rise to music and dance. BBS 44:e68. https://doi.org/10.1017/S0140525X20001144
Eleuteri V, Henderson M, Soldati A, Badihi G, Zuberbühler K, Hobaiter C. 2022. The form and function of chimpanzee buttress drumming. Anim Behav 192:189–205. https://doi.org/10.1016/j.anbehav.2022.07.013
Ettrup A, da Cunha-Bang S, McMahon B, Lehel S, Dyssegaard A, Skibsted A, et al. 2014. Serotonin 2A receptor agonist binding in the human brain with [¹¹C]Cimbi-36. J Cereb Blood Flow Metab 34(7):1188–1196. https://doi.org/10.1038/jcbfm.2014.68
Ettrup A, Svarer C, McMahon B, da Cunha-Bang S, Lehel K, Møller S, et al. 2016. Serotonin 2A receptor agonist binding in the human brain with [11C]Cimbi-36: Test-retest reproducibility and head-to-head comparison with the antagonist [18F]altanserin. Neuroimage 130:167–174. https://doi.org/10.1016/j.neuroimage.2016.02.001
Falk D. 2004. Prelinguistic evolution in early hominins: whence motherese? Behav Brain Sci 27(4):491–503; discussion 503–483. https://doi.org/10.1017/s0140525x04000111
Fazenda B, Scarre C, Till R, Pasalodos RJ, Guerra MR, Tejedor C, Ontañón Peredo R, Watson A, Wyatt S, Benito CG, Drinkall H, Fouldset F. 2017. Cave acoustics in prehistory: Exploring the association of Palaeolithic visual motifs and acoustic response. J Acoust Soc Am 142(3):1332–1349. https://doi.org/10.1121/1.4998721
Feenders G, Liedvogel M, Rivas M, Zapka M, Horita H, Hara E, et al. 2008. Molecular mapping of movement-associated areas in the avian brain: a motor theory for vocal learning origin. PLoS One 3(3):e1768. https://doi.org/10.1371/journal.pone.0001768
Fitch T. 2007. Evolving meaning: the roles of kin selection, allomothering and paternal care in language evolution. In: C Lyon, CL Nehaniv, A Cangelosi, editors. Emergence of Communication and Language. Springer: London, 29–51.
Fitch T. 2011. The evolution of syntax: an exaptationist perspective. Front Evol Neurosci 3:9.
Fleagle JG 1998. Primate Adaptation and Evolution: Elsevier Science. https://doi.org/10.3389/fnevo.2011.00009
Friberg A, Sundberg J. 1999. Does music performance allude to locomotion? A model of final ritardandi derived from measurements of stopping runners. J Acoust Soc Am 105(3):1469–1484. https://doi.org/10.1121/1.426687
Fuentes A, Kissel M, Spikins P, Molopyane K, Hawks J, Berger LR. 2023. Burials and engravings in a small-brained hominin, Homo naledi, from the late Pleistocene: contexts and evolutionary implications eLife 12:RP89125. https://doi.org/10.7554/eLife.89125.1
Geary D, Bailey DH, Oxford J. 2011. Reflections on the Human Family. In: TK Shackelford & C Salmon, editors. The Oxford Handbook of Evolutionary Family Psychology, Oxford University Press.
Geiringer K. 1982. Instrumente in der Musik des Abendlandes. Munich: Verlag CH Beck.
Ghazanfar AA, Takahashi DY. 2014. The evolution of speech: vision, rhythm, cooperation. Trends Cogn Sci 18(10):543–553. https://doi.org/10.1016/j.tics.2014.06.004
Glory A. 1964. La Grotte de Roucador. Bulletin de la Société Préhistorique Française 61:528–536.
Glory A. 1965. Nouvelles découvertes de dessins rupestres sur le Causse de Gramat (Lot). Bulletin de la Société préhistorique française. Études et travaux 62 (Fasc.3):528–538.
Goggi J, Pullar I, Carney SL, Bradford HF. 2002. Modulation of neurotransmitter release induced by brain-derived neurotrophic factor in rat brain striatal slices in vitro. Brain Res 941(1):34–42. https://doi.org/10.1016/s0006-8993(02)02505-2
Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. 2010. Prolactin, Oxytocin, and the development of paternal behavior across the first six months of fatherhood. Horm Behav 58(3):513–518. https://doi.org/10.1016/j.yhbeh.2010.04.007
Gordon CL, Cobb PR, Balasubramaniam R. 2018. Recruitment of the motor system during music listening: An ALE meta-analysis of fMRI data. PLOS ONE 13(11), e0207213. https://doi.org/10.1371/journal.pone.0207213
Grahn JA, Rowe JB. 2009. Neurosci J 29:7540–7548. Feeling the Beat: Premotor and Striatal Interactions in Musicians and Nonmusicians during Beat Perception. https://doi.org/10.1523/JNEUROSCI.2018-08.2009
Groenewoud F, Frommen JG, Josi D, Tanaka H, Jungwirth A, Taborsky M. 2016. Predation risk drives social complexity in cooperative breeders. Proc Natl Acad Sci U S A 113(15):4104–4109. https://doi.org/10.1073/pnas.1524178113
Hagen EH, Bryant GA. 2003. Music and dance as a coalition signaling system. Hum Nat 14(1):21–51. https://doi.org/10.1007/s12110-003-1015-z
Hahn J, Münzel S. 1995. Knochenflöten aus dem Aurignacien des Geißenklösterle bei Blaubeuren. Alb-Donaukreis.
Haile-Selassie Y, WoldeGabriel G. 2009. Ardipithecus Kadabba: Late Miocene Evidence from the Middle Awash, Ethiopia. University of California Press.
Hamilton WJ. 1982. Baboon sleeping site preferences and relationships to primate grouping patterns. Am J Primatol 3(1–4):41–53. https://doi.org/10.1002/ajp.1350030104
Hare B. 2017. Survival of the Friendliest: Homo sapiens Evolved via Selection for Prosociality. Annu Rev Psychol 68:155–186. https://doi.org/10.1146/annurev-psych-010416-044201
Hare B, Wobber V, Wrangham R. 2012. The self-domestication hypothesis: evolution of bonobo psychology is due to selection against aggression. Anim Beh 83 (3):573–585. https://doi.org/10.1016/j.anbehav.2011.12.007
Harmand S, Lewis JE, Feibel CS, Lepre CJ, Prat S, Lenoble A, et al. 2015. 3.3–million-year-old stone tools from Lomekwi 3, West Turkana, Kenya. Nature 521(7552):310–315. https://doi.org/10.1038/nature14464
Harrold FB. 1988. The Chatelperronian and the Early Aurignacian in France in The Early Upper Paleolithic. Evidence from Europe and the Near East. BAR. International Series (437):157–191.
Hart D, Sussman RW. 2011. The Influence of Predation on Primate and Early Human Evolution: Impetus for Cooperation. In: RW Sussman, CR Cloninger, editors. Origins of Altruism and Cooperation. New York, NY: Springer New York. 19–40.
Hart D, Sussman RW. 2019. Man the Hunted: Primates, Predators, and Human Evolution. Taylor & Francis Group.
Harvey AR. 2020. Links Between the Neurobiology of Oxytocin and Human Musicality. Front hum neurosci 14. https://doi.org/10.3389/fnhum.2020.00350
Hattori Y, Tomonaga M. 2020. Rhythmic swaying induced by sound in chimpanzees (Pan troglodytes). Proc Natl Acad Sci USA 117(2):936–942. https://doi.org/10.1073/pnas.1910318116
Hattori Y, Tomonaga M, Matsuzawa T. 2013. Spontaneous synchronized tapping to an auditory rhythm in a chimpanzee. Sci Rep 3:1566. https://doi.org/10.1038/srep01566
Hawkes K, Coxworth JE. 2013. Grandmothers and the evolution of human longevity: a review of findings and future directions. Evol Anthropol 22(6):294–302. https://doi.org/10.1002/evan.21382
Henneberg M, Eckhardt R. 2022. Evolution of modern humans is a result of self-amplifying feedbacks beginning in the Miocene and continuing without interruption until now. Anthropol Rev 85:77–83. https://doi.org/10.18778/1898-6773.85.1.05
Henneberg M, Saniotis A. 2016. Dynamic Human. Bentham Science Publishers.
Hilton CB, Moser CJ, Bertolo M, Lee-Rubin H, Amir D, Bainbridge CM, et al. 2022. Acoustic regularities in infant-directed speech and song across cultures. Nat Hum Behav 6(11):1545–1556. https://doi.org/10.1038/s41562-022-01410-x
Holloway RL. 1967. Tools and Teeth: Some Speculations regarding Canine Reduction. Am Anthropol 69(1):63–67.
Huyge D. 1990. Mousterian skiffle? Note on a Middle Palaeolithic engraved bone from Schulen, Belgium. Rock Art Research 7:125–132.
Huyge D. 1991. The ‘Venus’ of Laussel in the Light of Ethnomusicology. Archeologie in Vlaanderen.
Hrdy SB. 2009. Mothers and Others: The Evolutionary Origins of Mutual Understanding. Cambridge, Massachusetts: Belknap Press of Harvard University Press.
Hurt CP, Rice RR, McIntosh GC, Thaut MH. 1998. Rhythmic Auditory Stimulation in Gait Training for Patients with Traumatic Brain Injury. J Music Ther 35(4):228–241. https://doi.org/10.1093/jmt/35.4.228
Isler K, Van Schaik CP, 2012a. Allomaternal care, life history and brain size evolution in mammals. J Hum Evol 63:52–63. https://doi.org/10.1016/j.jhevol.2012.03.009
Iwamoto T, Mori A, Kawai M, Bekele A. 1996. Anti-predator behavior of gelada baboons. Primates 37(4)389–397. https://doi.org/10.1007/BF02381374
Jaubert J, Verheyden S, Genty D, Soulier M, Cheng H, Blamart D, et al. 2016. Early Neanderthal constructions deep in Bruniquel Cave in southwestern France. Nature, 534(7605):111–114. https://doi.org/10.1038/nature18291
Jordania J. 2009. Times to Fight and Times to Relax: Singing and Humming at The Beginnings of Human Evolutionary History. Kadmos, 252–276.
Jordania J. 2014. Tigers, Lions and Humans: History of Rivalry, Conflict, Reverence and Love. Logos.
Jordania J. 2020. Distribution of Singing in Arboreal and Terrestrial Species, with Implications for the Origins of Singing Behavior among Humans. Academia Letters. https://doi.org/10.20935/AL18.
Kalan AK, Carmignani E, Kronland-Martinet R, Ystad S, Chatron J, Aramaki M. 2019. Chimpanzees use tree species with a resonant timbre for accumulative stone throwing. Biol Lett 15(12):20190747. https://doi.org/10.1098/rsbl.2019.0747
Kimbel WH, Suwa G, Asfaw B, Rak Y, White TD. 2014. Ardipithecus ramidus and the evolution of the human cranial base. Proc Natl Acad Sci US 111(3):948–53. https://doi.org/10.1073/pnas.1322639111
Kivell TL, Schmitt D. 2009. Independent evolution of knuckle-walking in African apes shows that humans did not evolve from a knuckle-walking ancestor. Proc Natl Acad Sci 106(34):14241–14246. https://doi.org/10.1073/pnas.0901280106
Klein MO, Battagello DS, Cardoso AR, Hauser DN, Bittencourt JC, Correa RG. 2019. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell Mol Neurobiol 39(1):31–59. https://doi.org/10.1007/s10571-018-0632-3
Knight C, Lewis J. 2017. Wild Voices: Mimicry, Reversal, Metaphor, and the Emergence of Language. Curr Anthropol 58(4):435–453. https://doi.org/10.1086/692905
Kuhn L. Stiner MC. 1998. The Earliest Aurignacian of Riparo Mochi (Liguria, Italy). Curr Anthropol 39(S1):S175–S189. https://doi.org/10.1086/204694
Kumar G, Krishna R. 2014. Understanding the technology of the daraki-chattan cupules: The cupule replication project. Rock Art Res 31:177–186.
Kummer H, Hofer HO, Schultz AH, Starck D. 1967. Social Organization of Hamadryas Baboons: A Field Study: S. Karger AG.
Laland K, Wilkins C, Clayton N. 2016. The evolution of dance. Curr Biol 26(1):R5–R9. https://doi.org/10.1016/j.cub.2015.11.031
Lameira AR, Eerola T, Ravignani A. 2019. Coupled whole-body rhythmic entrainment between two chimpanzees. Sci Rep 9(1):18914. https://doi.org/10.1038/s41598-019-55360-y
Leach H. 2003. Human Domestication Reconsidered. Curr Anthropol 44(3):349–368. https://doi.org/10.1086/368119
Lemelin P, Schmitt D. 2016. On Primitiveness, Prehensility, and Opposability of the Primate Hand: The Contributions of Frederic Wood Jones and John Russell Napier. In: TL Kivell, P Lemelin, BG Richmond, D Schmitt, editors. The Evolution of the Primate Hand: Anatomical, Developmental, Functional, and Paleontological Evidence, New York, NY: Springer New York.
Lewis J. 2009. As well as words: Congo Pygmy hunting, mimicry, and play. In: R Botha, C Knight, editors. The Cradle of Language, Volume 2: African Perspectives. Oxford University Press.
Lieberman P. 2009. Human Language and Our Reptilian Brain: The Subcortical Bases of Speech, Syntax, and Thought. Harvard University Press.
Light KC, Grewen KM, Amico JA. 2005. More frequent partner hugs and higher oxytocin levels are linked to lower blood pressure and heart rate in premenopausal women. Biol Psychol 69(1)5–21. https://doi.org/10.1016/j.biopsycho.2004.11.002
Lin B, Foxfoot IR, Miller CM, Venkatamaran VV, Kerby JT, Bechtold EK, Kellogg BS, Nguyen N, Fashinget PJ. 2020. Leopard predation on gelada monkeys at Guassa, Ethiopia. Am J Primatol 82(2)e23098. https://doi.org/10.1002/ajp.23098
Locke JL. 2004. Trickle-up phonetics: A vocal role for the infant. Behav Brain Sci 27(4)516–516. https://doi.org/10.1017/S0140525X04360115
Locke JL, Bogin B. 2006. Language and life history: a new perspective on the development and evolution of human language. Behav Brain Sci 29:259–325. https://doi.org/10.1017/s0140525x0600906x
Lordkipanidze D. 2017. The History of Early Homo. In: M Tibayrenc, FJ Ayala, editors. On Human Nature. San Diego: Academic Press. 45–54.
Lordkipanidze D, Vekua A, Ferring R, Rightmire GP, Agusti J, Kiladze G, et al. 2005. Anthropology: The earliest toothless hominin skull. Nature 434(7034):717–718. https://doi.org/10.1038/434717b
Lordkipanidze D, Ponce de Leon MS, Margvelashvili A, Rak Y, Rightmire GP, Vekua A, et al. 2013. A Complete Skull from Dmanisi, Georgia, and the Evolutionary Biology of Early Homo. Science 342(6156)326–331. https://doi.org/10.1126/science.1238484
Lordkipanidze D, Agustí J, Rook L. 2023. Introduction to special issue: The biotic context of the Early Pleistocene hominins from Dmanisi (Georgia, southern Caucasus). J Hum Evol 174:103278. https://doi.org/10.1016/j.jhevol.2022.103278
Lovejoy CO, Latimer B, Suwa G, Asfaw B, White TD. 2009. Combining prehension and propulsion: the foot of Ardipithecus ramidus. Science 326(5949):72–72e8. https://doi.org/10.1126/science.117583
Lovejoy CO. 2009. Reexamining Human Origins in Light of Ardipithecus ramidus. Science 326(5949):74–74,74e1–74e8. https://doi.org/10.1126/science.1175834
Lovejoy CO, Simpson SW, White TD, Asfaw B, Suwa G. 2009. Careful climbing in the Miocene: the forelimbs of Ardipithecus ramidus and humans are primitive. Science 326(5949):70e1–8. https://doi.org/10.1126/science.1175827
Lundborg G. 2013. The Hand and the Brain: From Lucy’s Thumb to the Thought-Controlled Robotic Hand. Springer London.
Marean CW. 1989. Sabertooth cats and their relevance for early hominid diet and evolution. J Hum Evol 18(6)559–582. https://doi.org/10.1016/0047-2484(89)90018-3
Margvelashvili A, Tappen M, Rightmire GP, Tsikaridze N, Lordkipanidze D. 2022. An ancient cranium from Dmanisi: Evidence for interpersonal violence, disease, and possible predation by carnivores on Early Pleistocene Homo. J Hum Evol 166: 103180. https://doi.org/10.1016/j.jhevol.2022.103180
Maringer J. 1982. Musik und Musikinstrumente in vor-und frühgeschichtlicher Zeit. Praehist Z Berlin 57(1):126–137.
Martinowich K, Lu B. 2008. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacol 33(1):73–83. https://doi.org/10.1038/sj.npp.1301571
McPherron SP, Alemseged Z, Marean CW, Wynn JG, Reed D, Geraads D, Bobe R, Bearat HA. 2010. Evidence for stone-tool-assisted consumption of animal tissues before 3.39 million years ago at Dikika, Ethiopia. Nature 466(7308):857–860. https://doi.org/10.1038/nature09248
Meindl RS, Chaney ME, Lovejoy CO. 2018. Early hominids may have been weed species. Proc Natl Acad Sci 115(6)1244–1249. https://doi.org/10.1073/pnas.1719669115
Mithen S. 2009. The music instinct: the evolutionary basis of musicality. Ann N Y Acad Sci 1169:3–12. https://doi.org/10.1111/j.1749-6632.2009.04590.x
Mongle CS, Strait DS, Grine FE. 2019. Expanded character sampling underscores phylogenetic stability of Ardipithecus ramidus as a basal hominin. J Hum Evol 131:28–39. https://doi.org/10.1016/j.jhevol.2019.03.006
Montagu J. 2004. How Old Is Music? The Galpin Society Journal, 57,171–182. Available through JSTOR http://www.jstor.org/stable/25163800 [Accessed 30.05.2024].
Morley I. 2013. The prehistory of music: Human evolution, archaeology, and the origins of musicality. Oxford: Oxford University Press.
Moscovice LR, Surbeck M, Fruth B, Hohmann G, Jaeggi AV, Deschner T. 2019. The cooperative sex: Sexual interactions among female bonobos are linked to increases in oxytocin, proximity and coalitions. Horm Behav 116:104581. https://doi.org/10.1016/j.yhbeh.2019.104581
Napier J. 1962. The Evolution of the Hand. Sci Am 207(6):56–65. https://doi.org/10.1038/scientificamerican1262-56
Napier J. 1993. Hands. Princeton University Press.
Neubauer S, Hublin J, Gunz P. 2018. The evolution of modern human brain shape. Sci Adv 4(1):eaao5961. https://doi.org/10.1126/sciadv.aao5961
Nikolsky A, Benítez-Burraco A. 2022. Human aggression and music evolution: a model. Preprint PsyArXiv 245852812. https://doi.org/10.31234/osf.io/a8up7
O’Connell JF, Hawkes K, Blurton Jones NG. 1999. Grandmothering and the evolution of Homo erectus. J Hum Evol 36(5):461–485. https://doi.org/10.1006/jhev.1998.0285
Otte M. 1979. Le paléolithique supérieur ancien en Belgique. Musées royaux d’art et d’histoire.
Panksepp J. 2004. Affective Neuroscience: The Foundations of Human and Animal Emotions. Oxford University Press.
Panksepp J, Biven L. 2012. The Archaeology of Mind: Neuroevolutionary Origins of Human Emotions. WW Norton.
Panksepp J, Trevarthen C. 2009. The neuroscience of emotion in music. In: S Malloch, C Trevarthen, editors. Communicative musicality: Exploring the basis of human companionship. New York, NY, US: Oxford University Press.
Passemard E. 1944. La caverne d’Isturitz en Pays Basque, Préhistoire, t. Paris: Presses Universitaires de France.
Patel AD. 2021. Vocal learning as a preadaptation for the evolution of human beat perception and synchronization. Philos. Trans R Soc Lond B Biol Sci 376(1835)20200326. https://doi.org/10.1098/rstb.2020.0326
Pisor AC, Surbeck M. 2019. The evolution of intergroup tolerance in nonhuman primates and humans. Evol Anthropol 28(4)210–223. https://doi.org/10.1002/evan.21793
Plavcan JM, van Schaik CP. 1992. Intrasexual competition and canine dimorphism in anthropoid primates. Am J Phys Anthropol 87(4):461–477. https://doi.org/10.1002/ajpa.1330870407
Pontzer H, Antón SC, Lordkipanidze D. 2014. Dmanisi Hominins and Archaeology. In: Encyclopedia of Global Archaeology. Springer Nature 2146–2149.
Prang TC. 2019. The African ape-like foot of Ardipithecus ramidus and its implications for the origin of bipedalism. eLife 8:e44433. https://doi.org/10.7554/eLife.44433
Prang TC. 2022. New analyses of the Ardipithecus ramidus foot provide additional evidence of its African ape-like affinities: A reply to Chaney et al. (2021). J Hum Evol 164:103135. https://doi.org/10.1016/j.jhevol.2021.103135
Preston SD. 2013. The origins of altruism in offspring care. Psychol bull 139(6):1305. https://doi.org/10.1037/a0031755
Previc F. 1999. Dopamine and the Origins of Human Intelligence. Brain Cogn 41 (3):299–350. https://doi.org/10.1006/brcg.1999.1129
Previc F. 2002. Thyroid hormone production in chimpanzees and humans: Implications for the origins of human intelligence. Am J Phys Anthropol 118(4):402–403. https://doi.org/10.1002/ajpa.10095
Previc F. 2009. The Dopaminergic Mind in Human Evolution and History. Cambridge University Press.
Quam R, Martínez I, Rosa M, Bonmatí A, Lorenzo C, de Ruiter DJ, Moggi-Cecchi J, et al. 2015. Early hominin auditory capacities. Sci Adv 1(8). https://doi.org/10.1126/sciadv.1500355
Querejazu LR, Bednarik RG. 2010. Mysterious cup marks: proceedings of the First International Cupule Conference. BAR Publishing.
Raghanti MA, Edler MK, Stephenson AR, Munger EL, Jacobs B, Hof PR, Sherwood CC, Holloway RL, Lovejoyet CO. 2018. A neurochemical hypothesis for the origin of hominids. Proc Natl Acad Sci U S A 6:115(6):E1108–E1116 https://doi.org/10.1073/pnas.1719666115
Raghanti MA, Stimpson CD, Marcinkiewicz JL, Erwin JM, Hof PR, Sherwood CC. 2008. Cortical dopaminergic innervation among humans, chimpanzees, and macaque monkeys: A comparative study. Neurosci 155(1):203–220. https://doi.org/10.1016/j.neuroscience.2008.05.008
Raghanti MA, Sherwood CC. 2010. The evolution of cortical neurotransmitter systems among primates and their relevance to cognition. In: MYMD Broadfield, K Schick, NT Gosport, editors. The human brain evolving: paleoneurological studies in honor of Ralph L. Holloway. Stone Age Institute Press.
Raghanti MA, Edler MK, Stephenson AR, Wilson LJ, Hopkins WD, Ely JJ, Erwin JM, Jacobs B, Hof PR, Sherwood CC. 2016. Human-specific increase of dopaminergic innervation in a striatal region associated with speech and language: A comparative analysis of the primate basal ganglia. J Comp Neurol 524(10):2117–2129. https://doi.org/10.1002/cne.23937
Raglio AM. 2015. Music Therapy Interventions in Parkinson’s Disease: The State-of-the-Art. Front Neurol 6:185. https://doi.org/10.3389/fneur.2015.00185
Randall JA. 2015. Evolution and Function of Drumming as Communication in Mammals. Am Zool 4(5):1143–1156. https://doi.org/10.1093/icb/41.5.1143
Reno PL, Lovejoy CO. 2015. From Lucy to Kadanuumuu: balanced analyses of Australopithecus afarensis assemblages confirm only moderate skeletal dimorphism. PeerJ 3:e925. https://doi.org/10.7717/peerj.925
Richman B. 1987. Rhythm and melody in gelada vocal exchanges. Primates 28(2)199–223. https://doi.org/10.1007/BF02382570
Rilling JK, DeMarco AC, Hackett PD, Thompson R, Ditzen B, Patel R, Pagnoni G. 2012. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinol 37(4):447–461. https://doi.org/10.1016/j.psyneuen.2011.07.013
Rose MD. 1993. Locomotor anatomy of Miocene hominoids. In: DL Gebo, editor. Postcranial Adaptation in Nonhuman Primates. DeKalb, IL: Northern Illinois University Press. 252–272
Rosenberg KR, Golinkoff RM, Zosh JM. 2004. Did australopithecines (or early Homo) sling? Behav Brain Sci 27(4),522–522. https://doi.org/10.1017/S0140525X04430118
Salamone JD, Correa M. 2012. The mysterious motivational functions of mesolimbic dopamine. Neuron 76(3):470–485. https://doi.org/10.1016/j.neuron.2012.10.021
Samuni L, Langergraber KE, Surbeck, MH. 2022. Characterization of Pan social systems reveals in-group/out-group distinction and out-group tolerance in bonobos. Proc Natl Acad Sci U S A 119(26)e2201122119. https://doi.org/10.1073/pnas.2201122119
Samuni L, Preis A, Mundry R, Deschner T, Crockford C, Wittig RM. 2017. Oxytocin reactivity during intergroup conflict in wild chimpanzees. Proc Natl Acad Sci USA 114(2)268–273. https://doi.org/10.1073/pnas.1616812114
Saniotis A, Grantham JP, Kumaratilake J, Henneberg M. 2019. Neuro-hormonal Regulation Is a Better Indicator of Human Cognitive Abilities Than Brain Anatomy: The Need for a New Paradigm. Front Neuroanat 13:101. https://doi.org/10.3389/fnana.2019.00101
Saniotis A, Grantham JP, Kumaratilake J, Henneberg M, Mohammadi K. 2021. Going beyond brain size: An evolutionary overview of serotonergic regulation in human higher cortical functions. Anthropologie 59. https://doi.org/10.26720/anthro.20.08.10.1
Saniotis A, Henneberg M. 2011. An Evolutionary Approach Toward Exploring Altered States of Consciousness, Mind–Body Techniques, and Non-Local Mind. World Futures, 67(3)182–200. https://doi.org/10.1080/02604027.2011.555250
Saniotis A, Henneberg A. 2012. Craving for Drugs Is a Consequence of Evolution. Anthropos 107(2):571–578. Available through: JSTOR http://www.jstor.org/stable/23510061. [Accessed 30.05.2024].
Saniotis A, Henneberg A. 2013. Evolutionary Medicine and Future of Humanity: Will Evolution Have the Final Word? Hum Res 2:278–291. https://doi.org/10.3390/h2020278
Sayers K, Raghanti MA, Lovejoy CO. 2012. Human Evolution and the Chimpanzee Referential Doctrine. Ann Rev Anthropol 41(1),119–138. https://doi.org/10.1146/annurev-anthro-092611-145815
Schruth DM, Templeton CN, Holman DJ, Smith EA. 2024. The origins of musicality in the motion of primates. Am J Biol Anthropol 184(1)e24891. https://doi.org/10.1002/ajpa.24891
Senut B. 2006. Arboreal Origin of Bipedalism. In: H Ishida, R Tuttle, M Pickford, N Ogihara, M Nakatsukasa, editors. Human Origins and Environmental Backgrounds. Boston, MA: Springer US.
Senut B. 2015. The Miocene Hominoids and the Earliest Putative HominidsHominids. In: W Henke, I Tattersall, editors. Handbook of Paleoanthropology. Berlin, Heidelberg: Springer Berlin Heidelberg. 2043–2069.
Shove P, Repp B. 1995. Musical motion and performance: Theoretical and empirical perspectives. In: J Rink, editor. The Practice of Performance: Studies in Musical Interpretation. Cambridge: Cambridge University Press. 55–83. https://doi.org/10.1017/CBO9780511552366.004
Soubrié P. 1986. Reconciling the role of central serotonin neurons in human and animal behavior. Behav Brain Sci 9:319–335. https://doi.org/10.1017/S0140525X00022871
Sorato E, Gullett PR, Griffith SC, Russell AF. 2012. Effects of predation risk on foraging behaviour and group size: adaptations in a social cooperative species. Anim Behav 84(4)823–834. https://doi.org/10.1016/j.anbehav.2012.07.003
Stanner WEH. 2014. On Aboriginal Religion. Sydney University Press.
Sterelny K. 2012. The Evolved Apprentice. MIT Press.
Stout D, Hecht E, Khreisheh N, Bradley B, Chaminade T. 2015. Cognitive Demands of Lower Paleolithic Toolmaking. PLOS ONE 10(4):e0121804. https://doi.org/10.1371/journal.pone.0128256
Strehlow TGH. 1971. Songs of Central Australia. Angus and Robertson.
Suwa G, Asfaw B, Kono RT, Kubo D, Lovejoy CO, White TD. 2009. The Ardipithecus ramidus Skull and Its Implications for Hominid Origins. Science 326(5949):68–68, 68e1–68e7. https://doi.org/10.1126/science.1175825
Suwa G, Sasaki T, Semaw S, Rogers MJ, Simpson SW, Kunimatsu Y, et al. 2021. Canine sexual dimorphism in Ardipithecus ramidus was nearly human-like. Proc Natl Acad Sci U S A 118(49). https://doi.org/10.1073/pnas.2116630118
Thaut M. 2013. Rhythm, Music, and the Brain: Scientific Foundations and Clinical Applications. Taylor & Francis.
Thaut MH, McIntosh GC, Rice RR. 1997. Rhythmic facilitation of gait training in hemiparetic stroke rehabilitation. J Neurol Sci 151(2)207–212. https://doi.org/10.1016/s0022-510x(97)00146-9
Thaut MH, McIntosh KW, McIntosh GC, Hoemberg V. 2001. Auditory rhythmicity enhances movement and speech motor control in patients with Parkinson’s disease. Funct Neurol 16(2)163–172.
Thaut MH, Stephan KM, Wunderlich G, Schicks W, Tellmann L, Herzog H, McIntosh GC, Seitz RJ, Hömberg V. 2009. Distinct cortico-cerebellar activations in rhythmic auditory motor synchronization. Cortex 45(1)44–53. https://doi.org/10.1016/j.cortex.2007.09.009
Thaut MH, Tian B, Azimi-Sadjadi MR. 1998. Rhythmic finger tapping to cosine-wave modulated metronome sequences: Evidence of subliminal entrainment. Hum Mov Sci 17(6)839–863. https://doi.org/10.1016/S0167-9457(98)00031-1
Thin N. 1991. High spirits and heteroglossia: forest festivals of the Nilgiri Irulas. Doctoral thesis.
Thomas J, Kirby S. 2018. Self domestication and the evolution of language. Biol Philos 33 (1)9. https://doi.org/10.1007/s10539-018-9612-8
Tobias PV. 1971. The brain in hominid evolution / Phillip V. Tobias. Smithsonian Institution.
Treves A, Palmqvist P. 2007. Reconstructing Hominin Interactions with Mammalian Carnivores (6.0–1.8 Ma). In: SL Gursky and KAI Nekaris, editors. Primate Anti-Predator Strategies. Boston MA: Springer US.
Tsukahara T. 1993. Lions eat chimpanzees: The first evidence of predation by lions on wild chimpanzees. Am J Primatol 29(1)1–11. https://doi.org/10.1002/ajp.1350290102
Turk I. 1997. Mousterienska koščena piščal in druge najdbe iz Divjih Bab I v Sloveniji (Mousterian Bone Flute and other finds from Divje babe I Cave site in Slovenia). Opera Instituti Archaeologici Sloveniae 2:223.
Turk I, Dirjec J, Kavur B. 1995. Ali so v Sloveniji našli najstarejše glasbilo v Evropi?: The oldest musical instrument in Europe discovered in Slovenia? Slovenska akademija znanosti in umetnosti.
Turk M, Turk I, Dimkaroski L, Blackwell BAB, Horusitzky FZ, Otte M, Bastiani G, Korat L. 2018. The Mousterian musical instrument from the Divje Babe I Cave (Slovenia): Arguments on the material evidence for Neanderthal musical behaviour. L’anthropologie 122(4):679–706. https://doi.org/10.1016/j.anthro.2018.10.001
Tutin CEG, McGrew WC, Baldwin PJ. 1981. Responses of Wild Chimpanzees to Potential Predators. In: AB Chiarelli, RS Corruccini, editors. Primate Behavior and Sociobiology. Proc Life Sci Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-68254-4_19
Tutin CEG, McGrew WC, Baldwin PJ. 1983. Social organization of savanna-dwelling chimpanzees, Pan troglodytes verus at Mt. Assirik, Senegal. Primates 24(2)154–173. https://doi.org/10.1007/BF02381079
Vaultier M, Manuel Dos Santos F, Glory A. 1965. La grotte ornée d’Escoural, Portugal. Bulletin de la Société préhistorique française 62–1.110–117.
Vincent A. 1988. L’os comme artefact au Paléolithique moyen: principes d’étude et premiers résultats. L’homme de Néandertal 4:185–196.
Ward CV. 2013. Postcranial and Locomotor Adaptations of Hominoids. In: W Henke and I Tattersall, editors. Handbook of Paleoanthropology: Vol I:Principles, Methods and Approaches Vol II:Primate Evolution and Human Origins Vol III:Phylogeny of Hominids. Berlin, Heidelberg: Springer Berlin Heidelberg. 1–22.
Webb S. 2006. The First Boat People. Cambridge University Press.
White TD, Ambrose SH, Suwa G, Su DF, DeGusta D, Bernor RL, et al. 2009. Macrovertebrate paleontology and the Pliocene habitat of Ardipithecus ramidus. Science 326(5949):87–93. https://doi.org/10.1126/science.1175822
White TD, Asfaw B, Beyene Y, Haile-Selassie Y, Lovejoy CO, Suwa G, WoldeGabriel G. 2009. Ardipithecus ramidus and the paleobiology of early hominids. Science 326 (5949):75–86. https://doi.org/10.1126/science.1175802
White TD, Ambrose SH, Suwa G, WoldeGabriel G. 2010. Response to Comment on the Paleoenvironment of Ardipithecus ramidus. Science 328(5982),1105–1105. https://doi.org/10.1126/science.1185466
White TD, Lovejoy CO, Asfaw B, Carlson JP, Suwa G. 2015. Neither chimpanzee nor human, Ardipithecus reveals the surprising ancestry of both. Proc Natl Acad Sci USA 112(16):4877–4884. https://doi.org/10.1073/pnas.1403659111
Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama Y, Tutin C, Wrangham RW, Boesch C. 1999. Cultures in chimpanzees. Nature 399(6737):682–685. https://doi.org/10.1038/21415
Williams SA, Prang TC, Russo GA, Young NM, Gebo DL. 2023. African apes and the evolutionary history of orthogrady and bipedalism. Am J Biol Anthropol 181(S76)58–80. https://doi.org/10.1002/ajpa.24684
Wilson FR. 1999. The Hand: How Its Use Shapes the Brain, Language, and Human Culture. Vintage Books.
Wright E, Grawunder S, Ndayishimiye E, Galbany J, McFarlin SC, Stoinski TS, Robbins MM. 2021. Chest beats as an honest signal of body size in male mountain gorillas (Gorilla beringei beringei). Sci Rep 11(1):6879. https://doi.org/10.1038/s41598-021-86261-8
Zhou FC, Sari Y, Zhang JK. 2000. Expression of serotonin transporter protein in developing rat brain. Brain Res Dev Brain Res 119(1):33–45. https://doi.org/10.1016/s0165-3806(99)00152-2
Zuberbühler K, Jenny D, Bshary, R. 1999. The Predator Deterrence Function of Primate Alarm Calls. Ethology 105(6)477–490. https://doi.org/10.1046/j.14390310.1999.00396.x
Zubrow E, Blake E. 2006. The Origins of Music and Rhythm. In: Ch Scarre, Lawson G, editors. Archaeoacoustics. Cambridge: McDonald Institute Publications. 117–126.

