Available online at: https://doi.org/10.18778/1898-6773.87.1.04
 https://orcid.org/0000-0002-2721-2121
https://orcid.org/0000-0002-2721-2121
Department of Medical Biology, Pomeranian Medical University in Szczecin, Poland
 https://orcid.org/0009-0004-9544-4647
https://orcid.org/0009-0004-9544-4647
Department of Medical Biology, Pomeranian Medical University in Szczecin, Poland
 https://orcid.org/0000-0002-7513-8640
https://orcid.org/0000-0002-7513-8640
Department of Medical Biology, Pomeranian Medical University in Szczecin, Poland
ABSTRACT: North Sudan, especially the 4th Nile Cataract region, is home to one of the world’s most isolated human populations. This study aimed to clarify the ethnic background of the Shagia based on anthropological analyses. This study provides a morphological and ethnographic characterisation of the previously unstudied Shagia tribe. Head and body measurements were conducted among 64 adults from three villages. There were observable but relatively small admixture proportions of non-African population genes (light skin colour, narrow noses and masculine proportions) in their morphological build. The Shagia’s uniqueness may have been the result of severe genetic drift episodes resulting from founding events, such as long-term isolation and traditionally small population size. It is useful to trace the ethnic history of Africans and, specifically, for the tribal members of Shagia so that they may better understand and learn about their history. This study shows that the Shagia tribe was displaced from their territory due to the construction of the Merowe Dam. Thus, the results of this research fulfil the assumptions of urgent anthropology, as it contributes to the protection of the heritage of the 4th Nile Cataract region: an area of historical value to the study of the evolution of contemporary civilisation.
KEY WORDS: 4th Nile Cataract region, Shagia tribe, morphological structure, anthropometry, Sudan.
Africa is believed to be the cradle of humankind. One-eighth of the world’s population lives in Africa, primarily along the north and west coasts and along fertile river valleys. Sudan is one of the largest countries and is ethnically very diverse. Sudan was divided in 2011, which resulted in the creation of South Sudan as an independent country. This division was preceded by years-long armed conflicts between the north and south of the country. Before the division, the north was inhabited by Sudanese Arabs (49% of the total population) and the Kushites (the Beja and Nubians). In contrast, the south was inhabited by the Nilotes (Nuer, Dinka, Shilluk, etc.) and Sudanese tribes (the Azande and the Fur). Today, post-division, North Sudan is predominantly Muslim, while South Sudan is primarily Christian (Fadlalla 2004; Herman 2015; Cockett 2016).
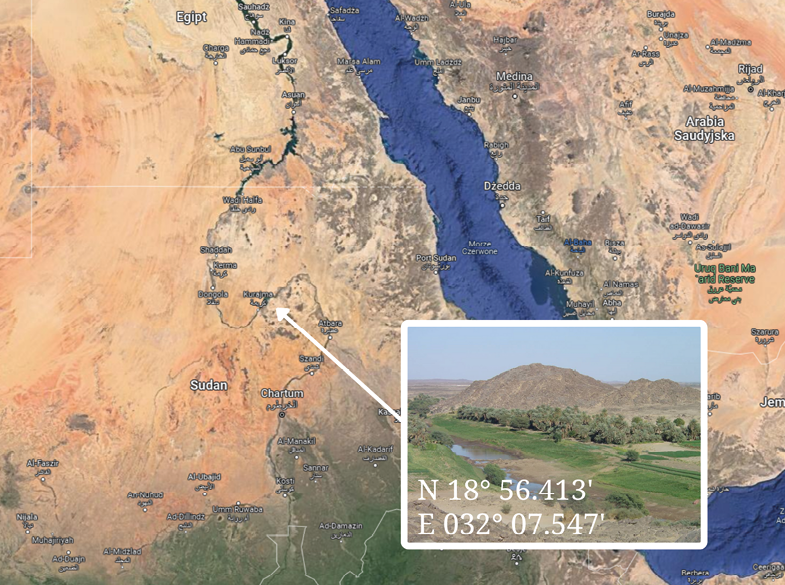
Prior to the division of Sudan, for centuries the northern region had been inhabited by a Semitic tribe, the Shagia. The 4th Nile Cataract region, due to its geographical location at the junction of two deserts (N 18º 56.413’ and E 032º 07.547’) (Fig. 1), left the Shagia tribe genetically and culturally isolated. The Shagia are known for both their nomadic and sedentary lifestyles. In the 4th Nile Cataract region, the Shagia are farmers, and their estates exist along the Nile, where families have lived together for generations. Each village consists of several farms and 40–60 people.
In 2005, as a consequence of the Merowe High Dam construction (Failer et al. 2006; Hildyard 2008), the possessions of numerous Shagia families were confiscated, and the people were displaced from the 4th Nile Cataract region. The government settled the Shagia in three locations in the Nubian Desert: Al-Multaqah, Al-Makabrab and Al-Muqadam and provided them with access to schools, mosques, hospitals, electricity and a primitive water supply system. However, the unrecorded history and culture of this tribe have been allowed to disappear. Therefore, the primary aim of this interdisciplinary research was to characterise the morphology of the Shagia people and provide an ethnographic description of their geographically, genetically, and culturally isolated population. Importantly, this research cannot be replicated as the Shagia villages disappeared once they were moved to multi-cultural settlements.
During a scientific expedition organised in February 2005 by the Museum of Archaeology in Gdańsk, Poland (before the displacement of Shagia from the 4th Nile Cataract region and the division of Sudan), anthropometric measurements were taken from individuals that occupied three villages (Abu Haras, Shibabit and El Higiena) (Fig. 1). A total of 64 adults underwent anthropological analysis. The study participants (n=29 men and 35 women, mean age = 23.9 ± 17.3 years, median age = 20 years) identified themselves as members of the Shagia tribe.
This research was conducted in accordance with the principles of the Declaration of Helsinki and local law. Informed consent was obtained from all participants. If study participants were unable to read, oral consent was obtained in the presence of a local government representative. The study received approval from the Bioethical Commission of the Pomeranian Medical University in Szczecin, Poland (KB–0012/129/10).

Fig. 1. Geographical location of Shagia tribe. (Photos, ©2023 TerraMetrics, Map data ©2023 Google)
The Shagia are farmers, and their homesteads are located along the Nile River, from Kurtia to the 3rd Cataract. Their spoken and written language is Arabic but there are few literate individuals in the sparsely populated villages. The houses are constructed of bricks made from mud or animal dung, and the roofs are thatched. The thatch provides insulation and helps to regulate the temperature inside the house by reducing heat transfer. The villages consist of farmsteads located near fields irrigated by the waters of the Nile. Each village is inhabited by 40–60 people. Families have multiple generations living together. The leaders are men in their prime (over 35 years of age) and are often not the family’s most senior members. Most work, including fieldwork, is performed by women. Newborns are given strings of colourful beads, which serve as amulets. Girls also have their ears pierced but, instead of earrings, they wear pieces of string due to impoverishment. Sometimes children undergo skin scarification in the cheek area, but this tradition is disappearing.
According to custom, girls are circumcised at the age of a few years (up to 5 years of age). The procedure includes clitoridectomies, as well as removing and sewing up the labia minora, and is an occasion celebrated by the entire village.
Our observations in the villages of Abu Haraz, Shibabit and El Higiena showed that the Shagia typically have a vegetarian diet consisting primarily of leguminous plants (beans, broad beans and crown vetch). While the country is rich in citrus fruits (grapefruits, oranges and lemons), they are rarely eaten because, due to their price, most Shagia cannot afford them. Vegetables cultivated in the fields are mainly Dutch tomatoes, onions and chives, and are the main sources of vitamins. On special occasions, such as weddings, christenings and the Ram Holiday, mutton or goat meat is eaten. The staple food is a flour-based bread called ‘gurassa’ and an overcooked broad bean called ‘ful’. After every meal, black tea with milk and sugar is drunk. An equally popular drink is an infusion of mint or chokeberry.
The faces of older adults exhibit scarification (Fig. 2), which takes the form of three horizontal cuts on the cheeks. Their bodies are decorated with henna, which is also used to underline the eyes of small children. Women may wear gold, which is a sign of prosperity.
There is a sharp divergence between having access to medical services and using them in practice. Doctors employed in medical centres (located in larger villages) are mainly those who are obliged to work in the provinces for several years after finishing their studies. They are responsible for administering vaccinations and providing prenatal and primary health care services. However, local people typically do not use the available for them services. Disease is narrowly defined among the Shagia people, with every non-traumatic pathology called malaria. The Shagia often use medicines received from citizens of Western countries, such as members of scientific expeditions. Local people use these medicines (without regard for contraindications and expiry dates) and treat every pill as a remedy. However, even doctors in health care centres may use antimalarial drugs for other diseases.
While Islam allows for polygamy, most men from the villages have only one wife due to a lack of wealth. On average, there are from one (young couples) to eleven children in a family (Fig. 3). The ratio of boys to girls is similar.
Our survey found a high degree of familial connections and consanguineal relationships between Abu Haraz villagers. However, despite the sparse population of Abu Haraz, our survey did not uncover marriages between close relatives.

Fig. 2. Face scarification in Shagia tribal members

Fig. 3. Shagia family
Body and head measurements were taken using anthropometric equipment in compliance with the principles of anthropometry (Malinowski et al. 2000; Tovee 2012). All measurements were performed using standard anthropological methodology based on standard anthropometric points using a medical scale, anthropometer, and digital or spring callipers. The measurement principles, recording method, and the instruments used were first developed by Martin (1928) and, with some modifications, are still widely used today (Malinowski et al. 2000; Tovee 2012). Measurements were labelled with the abbreviations of the anthropometric points that characterise them. Each participant assumed an anthropometric position (i.e., at attention, barefoot, with the upper limbs hanging freely along the trunk, hands close to the thighs and the head in the Frankfurt plane) for examination. Assuming this position allows for standardised measurements and observations during examinations and facilitates accurate comparisons and analyses across individuals.
The following cephalometric measurements (Farkas et al. 2005) were taken: head width (eu-eu), head length (g-op), minimum frontal breadth (ft-ft), face width (zy-zy), the morphological height of the face (n-gn), soft nose width (al-al) and nose height (n-ns).
Body height (B-v) was measured with an anthropometer with an accuracy of 0.1 cm. The following body measurements were taken: trunk length (sst-sy), biacromial (shoulder) width (a-a), biiliocristal width of the pelvis (ic-ic), diameter of the chest (chest depth) (xi-ths), chest width (thl-thl), arm length (a-daIII) and leg length (B-tro).
Somatic indices were calculated from the anthropometric measurements. The results of the individual ratios were assigned according to corresponding scales (Tab. 4, 5 and 6).
The cephalic index is an objective parameter for determining skull shape (Tab. 1). The minimum frontal-breadth / face-breadth index illustrates the ratio between the smallest breadth of the forehead and the width of the face. The facial index is a ratio of facial height to breadth and is used to determine various facial types. The nasal index differentiates sexual and ethnic changes and is an important measurement used in forensic science.
Rohrer’s index is an indication of a person’s weight relative to their height and is used as a proxy measure of adiposity. The trunk-length index shows the relative length of the trunk in relation to the total height. The acromial-height index indicates the relative width of the shoulders in relation to height, and the pelvic--height index shows the relative pelvic width to height. The arm-to-body ratio is defined as total arm length to total height, and the leg-to-body ratio is defined as the ratio of total leg length to total height (Versluys et al. 2018) (Tab. 2).
| Index | Classification | Range | |
| M | F | ||
| Cephalic index (by Martin-Saller) |
dolichocephalic | x – 75.9 | x – 76.9 |
| mesocephalic | 76.0 – 80.9 | 77.0 – 81.9 | |
| brachycephalic | 81.0 – 85.4 | 82.0 – 86.4 | |
| hyperbrachycephalic | 85.5 – x | 86.5 – x | |
| Minimum frontal-breadth / face-breadth index (by Lundborg-Linders and Saller) |
very narrow | x – 69.9 | x – 71.9 |
| narrow | 70.0 – 74.9 | 72.0 – 76.9 | |
| medium | 75.0 – 79.9 | 77.0 – 81.9 | |
| wide | 80.0 – 84.9 | 82.0 – 86.9 | |
| very wide | 85.0 – x | 87.0 – x | |
| Facial index (by Garson) |
hypereuroprosopic very wide | x – 78.9 | x – 76.9 |
| europrosopic wide | 79.0 – 83.9 | 77.0 – 80.9 | |
| mesoprosopic medium | 84.0 – 87.9 | 81.0 – 84.9 | |
| leptoprosopic narrow | 88.0 – 92.9 | 85.0 – 89.9 | |
| hyperleptoprosopic very narrow | 93.0 – x | 90.0 – x | |
| Nasal index (by Martin) |
very narrow | x – 54.9 | |
| narrow | 55.0 – 69.9 | ||
| medium | 70.0 – 84,9 | ||
| wide | 85.0 – 99.9 | ||
| very wide | 100.0 – x | ||
Indexes of sexual dimorphism were also calculated (Tab. 3) The pelvic-shoulder index is the relative width of the pelvis compared to the width of the shoulders, and the chest-flattening index shows the proportion of chest width to depth.
| Index | Classification | Range | |
| M | F | ||
| Pelvic-shoulder index (by Wanke (M) and Kolasa (F)) |
masculine proportions | x – 71.5 | x – 79.3 |
| intermediate proportions | 71.6 – 76.1 | 79.4 – 84.5 | |
| feminine proportions | 76.2 – x | 84.6 – x | |
| Chest-flattening index | very flat chest | x – 64.9 | x – 65.9 |
| flat chest | 65.0 – 68.4 | 66.0 – 68.9 | |
| medium flat chest | 68.5 – 71.4 | 69.0 – 70.9 | |
| barrel chest | 71.5 – 74.9 | 71.0 – 74.4 | |
| protruding chest | 75.0 – x | 74.5 – x | |
The somatic structure of each individual was determined using the Adam Wanke’s model. This is based on a comparison of the values of five indicators calculated for an individual to standard values characteristic of ‘pure’ types. The classification considers the body’s shape and the proportions of its various parts. Wanke characterised four body types labelled with the letters I, A, V and H. In this study, these values were presented according to the Wanke system for men and the Kolasa system for women (Malinowski and Bożiłow 1997).
The ethnographic method is only used in studies conducted in the natural environment (Angrosino 1997). This study used ethnographic research and participant observation. The ethnographic method involves collecting information about the values, beliefs, social relations, material goods of the community under study and provides a thorough description of the culture of the tribe. The current research applied ethnographic observations, which involved the regular recording of facts or events as a result of participating in tribal life over a period of time. Participant observation was in open form and supported by a photographic archive. All respondents gave their consent to be photographed.
Statistical analyses were performed using the STATISTICA software, version 9.0 (Statsoft, Inc., Hamburg, Germany). All data were categorised by gender, expressed as arithmetic means and presented as percentages.
Head and facial soft tissue measurements were conducted to determine reference values in the population. The results of the craniofacial anthropometric ratios for all 64 subjects are presented by gender in Table 4. The cephalic index indicated that the dolichocephalic (long head) type was most common among both men and women. The facial index classified Shagia faces as wide and very wide, with females having wider faces. The frontal breadth was classified as medium in the Shigia (in both men and women). Interestingly, given the climate they inhabited, they were characterised by narrow noses.
The mean height of the men was 170.08 cm; for women, it was 157.39 cm. The tallest man in Shibabit village was 188.4 cm, and the shortest (158 cm) came from Abu Haraz. The tallest woman was 171.4 cm, and the shortest was 146.1 cm. The Shagia men weighed, on average, 64.06 ± 10.14 kg, whilst women weighed 56.69 ± 11.21 kg. These measurements were also used to calculate body mass index (BMI). The BMI values showed that 17.24% of men and 28.57% of women were underweight, while 24.14% of men and 14.29% of women were overweight. Obesity was observed in 6.90% of men and 11.43% of women. Most of the population was well-nourished.
The most notable differences between the sexes were observed in Rohrer’s index. According to the averages for this index, men had a slender body build while women had a stout one. Residents of the villages were characterised by a short trunk, narrow shoulders and pelvis, with short lower and long upper limbs (Tab. 5).
| Index | Male N (29) |
Female N (35) |
||
| N | % | N | % | |
| Cephalic index(by Martin-Saller) | 18 | 62.07 | 18 | 51.43 |
| 10 | 34.48 | 13 | 37.15 | |
| 1 | 3.45 | 2 | 5.71 | |
| - | - | 2 | 5.71 | |
| Minimum frontal-breadth / face-breadth index(by Lundborg-Linders and Saller) | 1 | 3.45 | - | - |
| 3 | 10.34 | 4 | 11.43 | |
| 12 | 41.39 | 18 | 51.43 | |
| 10 | 34.48 | 11 | 31.43 | |
| 3 | 10.34 | 2 | 5.71 | |
| Facial index (by Garson) |
11 | 37.93 | 16 | 45.72 |
| 14 | 48.27 | 11 | 31.43 | |
| 1 | 3.45 | 3 | 8.57 | |
| 2 | 6.90 | 3 | 8.57 | |
| 1 | 3.45 | 2 | 5.71 | |
| Nasal index (by Martin) |
6 | 20.69 | 7 | 20.00 |
| 19 | 65.52 | 23 | 65.71 | |
| 4 | 13.79 | 5 | 14.29 | |
| - | - | - | - | |
| - | - | - | - | |
bold indicates the most common type
| Index | Male N (29) |
Female N (35) |
||
| N | % | N | % | |
| Rohrer Index (by Wanke (M) and Kolasa (F)) |
18 | 62.1 | 6 | 17.1 |
| 8 | 27.6 | 9 | 25.7 | |
| 3 | 10.3 | 20 | 57.2 | |
| Trunk-length index (by Wanke (M) and Kolasa (F)) |
27 | 93.1 | 31 | 88.6 |
| 2 | 6.9 | 3 | 8.6 | |
| - | - | 1 | 2.9 | |
| Acromial-height index (by Brugsch) |
20 | 69.0 | 25 | 71.4 |
| 6 | 20.7 | 7 | 20.0 | |
| 3 | 10.3 | 3 | 8.6 | |
| Pelvic-height index (by Vallois) |
20 | 69.0 | 20 | 57.2 |
| 7 | 24.1 | 4 | 11.4 | |
| 2 | 6.9 | 11 | 31.4 | |
| Arm-to-body ratio (by Vallois) |
8 | 27.6 | 13 | 37.2 |
| 9 | 31.0 | 9 | 25.7 | |
| 12 | 41.4 | 13 | 37.2 | |
| Leg-to-body ratio (by Malinowski) |
11 | 37.9 | 14 | 40.0 |
| 9 | 31.0 | 9 | 25.7 | |
| 9 | 31.0 | 12 | 34.3 | |
bold indicates the most common type
Genetic sexual dimorphism (Tab. 6) influenced the bi-directional development of morphological and physiological traits. Sexual variation in body shape is highly dependent on physical workloads, and the Shagia had protruding chests and masculine proportions.
Body types (according to the Wanke method) are presented in Figure 4. Among men, the type I body build was the most common. Type I is characterised by an i.a. narrow shoulders. Whereas among women, the most common was type H, which is characterised by an i.a. short trunk and protruding chest. It is rare to find a pure type. Typically, only if the percentage is above 68% can the individual be classified as pure. Otherwise, individuals are considered mixed. Among men, a mixed I-V type body build was the most common; among women, it was type H-V.
| Index | Male N (29) |
Female N (35) |
||
| N | % | N | M | |
| Pelvic-shoulder index (by Wanke (M) and Kolasa (F)) |
12 | 41.4 | 24 | 68.6 |
| 6 | 20.7 | 5 | 14.3 | |
| 11 | 37.9 | 6 | 17.1 | |
| Chest-flattening index | 2 | 6.9 | 1 | 2.9 |
| 1 | 3.5 | 7 | 20.0 | |
| 3 | 10.3 | 2 | 5.7 | |
| 4 | 13.8 | 5 | 14.2 | |
| 19 | 65.5 | 20 | 57.2 | |
bold indicates the most common type
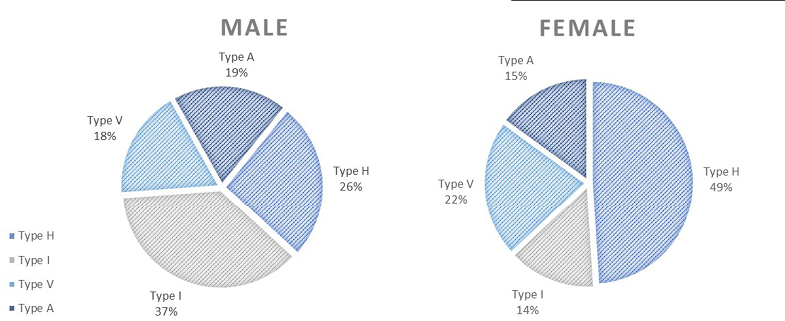
Fig. 4. Body types according to the Wanke method
The difficult conditions of the natural environment influenced the Shagia’s morphological build, while deeply rooted tribal affiliation helped maintain characteristic features (with relatively small admixture proportions for non-African genes) for this indigenous ethnic group.
Members of the Shagia tribe have long heads, either wide or very wide faces, mid-sized or wide foreheads, and narrow noses. Interestingly, narrow noses naturally occur in dry and cold climates due to natural selection (Coop et al. 2009), and this nasal shape is most common in individuals from East Asia (Dhulqarnain et al. 2020). Specific conditions of their bio-geographical environment have likely caused the characteristic somatic build of the Shagia. Residents of the villages were of medium height. The mean value of the pelvic-shoulder index in the Shagia was the same as men of Egyptian origin, who have a lighter skin complexion that is associated with Caucasian or European ancestry. Narrow hips and relatively long lower limbs, accompanied by short height, are likely related to bio-geographical origin and are typical of individuals whose ancestry is primarily from the African continent and possess dark skin pigmentation, regardless of their gender and geographical location.
Among the Shagia from the 4th Cataract region, some anthropometric characteristics indicated a bio-geographical origin from East Asia and the Mediterranean region. Anthropometric features characteristic of East Asians among the examined individuals of the Shagia can be explained historically. In pre-Islamic times, trade routes from Aksum and the Red Sea crossed at Meroë, and ships sailing from Arabia to Lake Chad to Kanem anchored in the Red Sea. The first migration of southern Arabian tribes from Arabia to East Africa occurred in 1000 BC, as recorded in the Sabanese inscriptions of South Arabia and Aksum. At the beginning of the new era, there was heavy migration to Nubia, Kurdufan and Darfur via Axum and Atbara along the Blue Nile (Fadlalla 2004).
It is unclear what role body proportions play in the bio-geographical diversity of modern humans. However, for the isolated Shagia, who have not been previously described anthropologically, anthropometry can provide clues to explain the history of the group. Moreover, recent advancements in genetic research have provided additional insights into human variation that complement anthropometric studies (Rindos 1992). In our previous research (Kempińska-Podhorodecka et al. 2008), we found a single delta32 allele of the human chemokine receptor 5 (CCR5) gene in the Shagia population, which is consistent with other studies investigating the rarity of this allele in African populations (Galvani and Novembre 2005). This allele is a 32-base-pair deletion that results in natural resistance to HIV infection among delta32 homozygotes (Glass et al. 2006). In the Shagia, the mutation may be related to their nomadic lifestyle and possible European admixture.
Similarly, the genetic polymorphisms of the Duffy antigen receptor for chemokine genes, which successfully protect against blood-stage infection by Plasmodium vivax (Donahue et al. 1968), has been identified in Shagia tribes and may also be a consequence of admixture with non-Africans (Kempinska-Podhorodecka et al. 2012a). This is consistent with the anthropological data and is likely due to the free flow of genetic material in the population. The hypothesis that Shagia individuals were separated from other tribes in the region of the 4th Nile Cataract is also consistent with studies showing genetic variance in glucose dehydrogenase 6-phosphate (G6PD) that, in humans, determines the response to malaria exposure (Kempinska-Podhorodecka et al. 2013). The identification of a single G6PD mutation in the Shagia suggests that there was long-term assimilation with other tribes. It should be emphasised that the culture of today’s Sudanese tribes is not homogeneous because even before the arrival of Arabs, indigenous populations demonstrated a vast array of customs and languages. Thus, the impact they made on newcomers would have been varied. However, it is surprising that the Shagia people of today do not identify with other tribes (Hildyard 2008) in Sudan. Hence, the focus on small and isolated populations can provide important insights into the factors affecting the distribution of heritable traits in Africa.
The results of our previous genetic studies (Kempińska-Podhorodecka et al. 2008; 2011; 2012a; 2012b; 2013; 2014) showed that Shagia individuals were separated from other tribes in the region of the 4th Cataract. These genetic results are consistent with the anthropological data and suggest an admixture of non-African populations over specific periods (Campbell and Tishkoff 2008; Lambert and Tishkoff 2009). However, the lack of genetic variation observed in this population may be due not only to the ethnic roots of the Shagia people but also to the geographical isolation of the villages. This can be partly explained by the familial relationships in the Abu Haras village, which had a high degree of consanguinity among the inhabitants (marriage only within their tribes). Cultural conditioning favoured intra-village marriages that were virtually free of extraneous genetic material. However, it is essential to approach the study of human variation with caution, as it should not be used to perpetuate stereotypes or discriminatory practices. It is vital to consider individual variation and the complex interplay of genetic, environmental and cultural factors that contribute to human differences.
It is difficult to clearly establish the ethnic affiliation of the Shagia tribe due to their nomadic lifestyle and historic assimilation with other populations. On the one hand, this obstructed access to the population. On the other hand, these features were a prerequisite to the population’s uniqueness. Furthermore, our analysis of the Shagia people from three isolated villages has made it possible to preserve their cultural heritage. Following this study, the Shagia tribe was displaced from their territory due to the construction of the Merowe Dam. As a result, their homeland was flooded with a newly formed lake. Thus, the Shagia in these villages migrated to various areas occupied by other tribes, preventing the possibility of further research on the Shagia people.
The available literature provides little information about the Shagia, and the existing reports are usually historical. Unfortunately, this information gap is difficult to fill given that, following resettlement, the Shagia have been living with populations of thousands of individuals from various tribes and have established new social orders, traditions and cultures.
Overall, this study characterises a previously unexplored ethnic group in Sudan and provides important insights into the anthropological diversity of the local population. Human diversity is of immense importance to scientists for the effective diagnosis and treatment of diseases and for classifying people based on ethnicity. It should be also noted that the villages of the Shagia have never been mapped. Thus, from a scientific perspective, the Shagia are interesting because they were a homogeneous population resulting from their geographical, genetic, and cultural isolation. The Shagia constitute an example of how genetic attributes may mirror morphology and socio-cultural features in human populations. Furthermore, our findings are valuable for tribal members to understand and learn about their history.
Acknowledgements
The authors wish to thank Mahmoud el Tayeb and the entire Shagia tribe for their support and kindness. In addition, we would like to express our gratitude to the reviewer of this paper for valuable comments.
Authors’ contribution
Conceptualisation, A.K.-P.; methodology, A.S. and A.L.; validation, A.K.-P.; formal analysis, A.S., A.L. and A.K.-P.; investigation, A.S. and A.K.-P.; resources, A.K.-P.; data curation, A.S.; writing original draft preparation, A.S. and A.K.-P.; writing review and editing, all authors; visualisation, A.S., A.L. and A.K.-P.; supervision A.K.-P.; project administration, A.K.-P.; funding acquisition, A.K.-P. All authors have read and agreed to the published version of the manuscript.
Funding
N N404 140837
Declarations of interest
The authors declare no conflict of interest.
Ethical statement
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Bioethical Commission of the Pomeranian Medical University in Szczecin, Poland (KB-0012/129/10).
Angrosino M. 2010. Badania etnograficzne i obserwacyjne przeł. M. Brzozowska-Brywczyńska, Warszawa.
Campbell MC, Tishkoff SA. 2008. African Genetic Diversity: Implications for Human Demographic History, Modern Human Origins, and Complex Disease Mapping. Annu Rev Genomics Hum Genet 9:403–33. https://doi.org/10.1146/annurev.genom.9.081307.164258
Cockett R. 2016. Sudan: The Failure and Division of an African State. Yale University Press.
Coop G, Pickrell JK, Novembre J, Kudaravalli S, Li J, Absher D, Myers RM, Cavalli-Sforza LL, Feldman MW, Pritchard JK. 2009. The Role of Geography in Human Adaptation. PLoS Genet 5(6):e1000500. https://doi.org/10.1371/journal.pgen.1000500
Dhulqarnain AO, Mokhtari T, Rastegar T, Mohammed I, Ijaz S, Hassanzadeh G. 2020. Comparison of Nasal Index between Northwestern Nigeria and Northern Iranian Populations: An Anthropometric Study. J Maxillofac Oral Surg 19(4):596–602. https://doi.org/10.1007/s12663-019-01314-w
Donahue RP, Bias WB, Renwick JH, McKusick VA. 1968. Probable Assignment of the Duffy Blood Group Locus to Chromosome 1 in Man. Proc Natl Acad Sci USA 61(3):949–55.
Fadlalla MH. 2004. Short History of Sudan. Iuniverse, Inc. New York, USA.
Failer E, Mutaz M, El Tayeb A. 2006. Merowe: The Largest Water Resources Project under Construction in Africa. Int J Hydropower Dams 13(6):68.
Farkas LG, Katic MJ, Forrest CR, Alt KW, Bagic I, Baltadjiev G, Cunha E, Cvicelova M, Davies S, Erasmus I, Gillett-Netting R, Hajnis K, Kemkes-Grottenthaler A, Khomyakova I, Kumi A, Kgamphe JS, Kayo-Daigo N, Le T, Malinowski A, Negasheva M, Manolis S, Ogeturk M, Parvizrad R, Rosing F, Sahu P, Sforza C, Sivkov S, Sultanova N, Tomazo-Ravnik T, Toth G, Uzun A, Yahia E. 2005. International Anthropometric Study of Facial Morphology in Various Ethnic Groups/Races. J Craniofac Surg 16(4):615–46. https://doi.org/10.1097/01.scs.0000171847.58031.9e
Galvani AP, Novembre J. 2005. The Evolutionary History of the Ccr5-Δ32 Hiv-Resistance Mutation. Microbes Infect 7(2):302–09.
Glass WG, McDermott DH, Lim JK, Lekhong S, Yu SF, Frank WA, Pape J, Cheshier RC, Murphy PM. 2006. Ccr5 Deficiency Increases Risk of Symptomatic West Nile Virus Infection. J Exp Med 203(1):35–40.
Herman JL. 2015. Trauma and Recovery: The Aftermath of Violence – from Domestic Abuse to Political Terror. Hachette UK.
Hildyard N. 2008. Neutral? Against What? Bystanders and Human Rights Abuses: The Case of Merowe Dam. Sudan Stud 37:19–38.
Kempinska-Podhorodecka A, Knap O, Drozd A, Kaczmarczyk M, Parafiniuk M, Parczewski M, Ciechanowicz A. 2012a. Analysis for Genotyping Duffy Blood Group in Inhabitants of Sudan, the Fourth Cataract of the Nile. Malar J 11:115. https://doi.org/10.1186/1475-2875-11-115
Kempinska-Podhorodecka A, Knap O, Drozd A, Kaczmarczyk M, Parafiniuk M, Parczewski M, Milkiewicz M. 2013. Analysis of the Genetic Variants of Glucose-6-Phosphate Dehydrogenase in Inhabitants of the 4th Nile Cataract Region in Sudan. Blood Cells Mol Dis 50(2):115–18. http://doi.org/10.1016/j.bcmd.2012.10.003
Kempinska-Podhorodecka A, Knap O, Popadowska A, Drozd A. 2014. An Association between Lactose Intolerance and Anthropometric Variables in the Sudanese Shagia Tribe (East Africa). Ann Hum Biol 41(5):460–64. http://doi.org/10.3109/03014460.2013.877965
Kempinska-Podhorodecka AD, Knap OM, Kobus K, Ciechanowicz A. 2012b. Frequencies of Functional Caspase 12 Genotypes in the North-Africa Population. Russ J Genet 48(4):477–79. http://doi.org/10.1134/S1022795412030040
Kempinska-Podhorodecka A, Knap OM, Parczewski M, Bińczak-Kuleta A, Parafiniuk M. 2008. Report on the D32 Ccr5 Variant in the Sudanese Shagia Tribe. Anthropol Rev 71:71–76.
Kempinska-Podhorodecka A, Knap OM, Parczewski M, Bińczak-Kuleta A, i Ciechanowicz A. 2011. Sickle Cell Anemia-Associated Beta-Globin Mutation in Shagia and Manasir Tribes from Sudan. Pol J Environ Stud 20(6).
Lambert CA, Tishkoff SA. 2009. Genetic Structure in African Populations: Implications for Human Demographic History. Evolution: The Molecular Landscape 74:395–402. http://doi.org/10.1101/sqb.2009.74.053
Malinowski A, Bożiłow W. 1997. Podstawy antropometrii: metody, techniki, normy. Wydawnictwo Naukowe PWN.
Malinowski A, Stolarczyk H, Lorkiewicz W. 2000. Antropologia a medycyna i promocja zdrowia. Wydawnictwo Uniwersytetu Łódzkiego.
Rindos D. 1992. Coevolution – Genes, Culture, and Human-Diversity – Durham,Wh. Am J Phys Anthropol 88(2):265–67. http://doi.org/10.1002/ajpa.1330880215
Versluys TMM, Foley RA, Skylark WJ. 2018. The influence of leg-to-body ratio, arm-to-body ratio and intra-limb ratio on male human attractiveness. R Soc Open Sci 5(5):171790.
Tovee M. 2012. Anthropometry In: T Cash editor. Encyclopedia of Body Image and Human Appearance. Elsevier Inc.

