Introduction
Decreasing mineral density of skeleton bones is a natural involutionary process, which may lead to osteoporosis (i.e., excessively low bone mass with a simultaneously maintained proportion between the organic and the mineral fraction) and, ultimately, to enhanced bone fragility. The main factors that significantly increase the risk of osteoporosis among postmenopausal women include low peak bone mass (PBM, achieved, on the average, at the age of 18 years; Roy et al. 2005), late menarche age (McKay et al. 1998) and menopause with accompanying declines in oestrogen levels. The most common environmental determinants of osteoporosis are low body mass index (BMI), irregular nutrition, diet poor in calcium and vitamin D, sedentary lifestyle, alcohol abuse and tobacco smoking (Cooper et al. 2006; Sinaki 2007; Wilsgaard et al. 2009; Özbaş et al. 2012). The body weight and BMI are important factors affecting BMD of women at the age of peak bone mass (Henderson et al. 1995; Al Rassy et al. 2018) and in postmenopausal females (Ravn et al. 1999; Wu & Du 2016). An increased BMI (and the resulting higher fat mass) has a protective impact on bone density (Barrera et al. 2004), and, depending on the fat distribution in pre- and postmenopausal females (Fu et al. 2011), correlates with endocrine alterations. The latter positively influences bone metabolism (Zhao et al. 2008), predisposing to higher BMD, thicker and denser cortices, and higher trabecular number (Evans et al. 2015). At a population level, high BMI remains a protective factor for most sites of fragility fracture (Johansson et al. 2014). Thinness (low percentage of body fat, low BMI, or low body weight) predisposes postmenopausal females to rapid bone loss, low bone mass, osteoporosis and related fracture risk (Ravn et al. 1999; Kanis et al. 2011; Prieto-Alhambra et al. 2012), which is mediated by the interaction between BMI and BMD (Johansson et al. 2014).
Genetic factors are also significant. The genes, whose polymorphisms significantly correlate with an increased risk of osteoporosis, include, among others: oestrogen receptor (ER), transforming growth factor beta 1 (TGF-β1), interleukin 10 (IL-10), interleukin 6 (IL6), interleukin 17-F (IL17F), vitamin D receptor (VDR), cytochrome P-450c17alpha (CYP17), plasminogen activator inhibitor-1 (PAI-1), collagen type I alpha 1 (COL1A-1) and calcitonin receptor (CALCR) (Chen et al. 2005; Bustamante et al. 2007; Seremak-Mrozikiewicz et al. 2009; Oishi et al. 2012; Tural et al. 2013).
Some studies also indicate prenatal risk factors of osteoporosis, as well as osteoporosis-related, higher incidence of femoral bone fractures (Cooper et al. 2009). There is some evidence suggesting that the peak bone mass might be heritable although the current genetic markers are able to account only for a small proportion of individual bone mass variation or fracture risks. The mechanism of this relation reveals an intrauterine control mechanism of neonatal skeletal growth and mineralization. This mechanism appears to be mediated by modulation of the set-point for basal activity of a pituitary-dependent endocrine system, such as the hypothalamic-pituitary-adrenal (HPA) and the growth hormone/insulin-like growth factor-1 (GH/IGH-1) axes (Godfrey et al. 2001; Javaid et al. 2006; Cooper et al. 2009). According to Godfrey et al. (2001), the neonatal bone mass is significantly and positively correlated with childbirth parameters (body weight and length), standardised for sex and pregnancy duration and by weight of the placenta alone. Maternal factors, which significantly and negatively correlate with offspring bone mass content (BMC), include maternal smoking and maternal energy intake at 18 weeks of gestation (Godfrey et al. 2001), as well as reduced maternal height, lower pre-conception maternal weight, reduced maternal fat stores during late pregnancy and lower maternal social class (Javaid et al. 2006).
Birth season is a derivative of the prenatal development season. In European populations, the birth season significantly correlates with many biological features that is observed both at the population and individual level, including fecundity, conception and birth (Lam et al. 1994), sex ratio at birth (Nonaka et al. 1999), childbirth parameters (Chodick et al. 2009), infant and adult mortality (Doblhammer & Vaupel 2001), body height and body weight in later life (Krenz-Niedbała et al. 2011), cardiovascular conditions in adulthood (Doblhammer & Vaupel 2001), life expectancy and the probability of death at older ages (Doblhammer & Vaupel 2001; Doblhammer et al. 2005), the incidence of certain neurodegenerative diseases, such as multiple sclerosis (Salzer et al. 2010) and Parkinson’s disease (Gardener et al. 2010), as well as the incidence rates of certain mental diseases, such as schizophrenia and psychotic-like experiences (Tochigi et al. 2013).
Studies on Polish population demonstrate a significant relationship of the birth season with biological features, such as birth body length (Siniarska & Kozieł 2010), birth body height and body weight in later life (Krenz-Niedbała et al. 2011), the width of enamel layer of deciduous teeth (Żądzińska et al. 2013), as well as the incidence of the nervous system diseases, including cerebral palsy (Kulak & Sobaniec 2005).
Although the number of studies analysing the relationship between the prenatal development environment (including the birth season) with neonatal bone mineral density (BMD) and neonatal bone mineral content is fairly high (Namgung et al. 1998; Godfrey et al. 2001; Javaid et al. 2006), the number of reports, indicating “maintenance” of this relationship in adult life is rather low. Some notable exceptions from the mentioned above relationship include studies regarding birth season to significantly increase the risk of osteoporosis-related femoral bone fractures in Danish men and women at the age above 65 (Abrahamsen et al. 2012), and a study conducted on the Norwegian population that indicated that the month of birth significantly correlates with radiographically diagnosed bilateral hip and knee arthrosis (Fønnebø 1995). It is thus possible that the observed clinical effects of bone metabolism disorders are partly a consequence of bone mineral density modified by the season of prenatal development (the birth season). The study concerns women at the perimenopausal age in whom, while ageing, the natural phenomenon of gradual bone density loss in the lumbar section of the spine occurs. The aim of this study was to evaluate a relationship between the birth season and the level of bone mineral density in Polish women in perimenopausal age.
Material and methods
Study participants
A total of 653 Polish women at the age of 50.0–59.9 years were included in the study, all of them being first-time patients, attending the Outpatient Clinic of Osteoporosis at the Medical University Hospital in Łódź (Poland) during the years 2002–2015. All the patients were at that time residents of Łódź – a city located in central Poland with the population of 722 thousand inhabitants. The study was approved by the Institutional Bioethical Committee of the University of Łódź. A written informed consent was obtained from all study participants.
The mean age of examined women was 55.80±2.55 years, the mean weight: 67.37±12.07 kg, the mean height: 161.28±5.67 cm, and the mean body mass index (BMI): 25.88±4.29 kg/m2. Birth season was defined as follows: spring – women, born from the 1st of March through the 31st of May; summer – women, born from the 1st of June through the 31st of August; autumn – women, born from the 1st of September through the 30th of November; winter – women, born from the 1st of December through 29th of February.
Bone mineral density measurements
BMD measurements were performed at the Outpatient Clinic of Osteoporosis, Medical University Hospital of Łódź. BMDs of the lumbar spine were measured by dual-energy x-ray absorptiometry (Lunar Prodigy, GE Lunar, Madison, WI, USA) at medium 750 µA scan mode. Lumbar spine scans were obtained with patient on table in supine position, adhering to the manufacturer’s protocols. Quality control scans, carried out during a 4-year follow-up period, indicated no gear-related shifts in BMD levels. Statistical analyses were based on measured lumbar BMD values (g/cm2) for L1, L2, L3, L4, L1-L2, L1-L3, L1-L4, L2-L3, L2-L4 and L3-L4.
Statistical analysis
All studied variables were evaluated for normality using the Shapiro-Wilk test and for equality of variance using the Levene’s test. The associations of the studied variables (age, BMI, and BMD measurements of the lumbar section of the spine) were assessed with a non-parametric correlation test (Spearman’s R). Age and BMI of the examined women by season of birth were compared using non-parametric equivalent of ANOVA (Kruskal-Wallis test). To eliminate the influence of age and BMI on BMD, we used the multiple regression-dependent variable: BMD (g/cm2) measurements of lumbar section of the spine; the independent variables: age (years) and BMI (kg/m2). Thus, we considered the residuals as age- and BMI-independent measures of BMD. The residuals were calculated separately for measurement of lumbar section of the spine (resL1, resL2, etc.). They were used as dependent variables to assess the diversity of the BMD values according to the season of birth of women using the analysis of one-way variance (ANOVA) with the Bonferroni post-hoc test. All the statistical analyses were performed using the STATISTICA software (TIBCO Software Inc., version 13).
Results
Age of female study participants was not significantly correlated with their BMI (R=0.034; p=0.392). However, the BMD values of the lumbar spine showed significant and negative correlation with age (p<0.001) and positive correlation with BMI (p<0.001) – Table 1.
The birth season did not differentiate the examined women by age (H=2.43; p=0.488) and BMI (H=0.75; p=0.861) – Table 2. Over half of the women were born in spring and summer (56.2%).
|
BMD (g/cm2) measurements of the lumbar section of the spine |
Age (years) & BMD (g/cm2) |
BMI (kg/m2) & BMD (g/cm2) |
||||
| Mean | SD | R | p value | R | p value | |
| L1 | 0.923 | 0.151 | -0.213 | <0.001 | 0.262 | <0.001 |
| L2 | 0.981 | 0.165 | -0.213 | <0.001 | 0.282 | <0.001 |
| L3 | 1.041 | 0.176 | -0.183 | <0.001 | 0.263 | <0.001 |
| L4 | 1.024 | 0.195 | -0.136 | <0.001 | 0.262 | <0.001 |
| L1-L2 | 0.953 | 0.154 | -0.218 | <0.001 | 0.283 | <0.001 |
| L1-L3 | 0.985 | 0.159 | -0.209 | <0.001 | 0.282 | <0.001 |
| L1-L4 | 0.996 | 0.164 | -0.191 | <0.001 | 0.283 | <0.001 |
| L2-L3 | 1.013 | 0.167 | -0.201 | <0.001 | 0.278 | <0.001 |
| L2-L4 | 1.017 | 0.172 | -0.181 | <0.001 | 0.279 | <0.001 |
| L3-L4 | 1.032 | 0.180 | -0.162 | <0.001 | 0.269 | <0.001 |
BMI, body mass index; BMD, bone mineral density; SD, standard deviation; R and p value, non-parametric correlation test (Spearman’s R).
| Season of birth | n | % | Age (years) | BMI (kg/m2) | ||||
| Median | Q1 | Q3 | Median | Q1 | Q3 | |||
| Spring | 182 | 27.9 | 56.21 | 54.18 | 58.25 | 25.84 | 23.06 | 28.63 |
| Summer | 185 | 28.3 | 55.99 | 53.39 | 57.69 | 25.08 | 22.83 | 28.63 |
| Autumn | 152 | 23.3 | 55.93 | 53.92 | 57.97 | 25.23 | 22.86 | 28.16 |
| Winter | 134 | 20.5 | 56.03 | 54.00 | 57.78 | 25.45 | 23.23 | 28.44 |
| Kruskal-Wallis test | H = 2.43; p = 0.488 | H = 0.75; p = 0.861 | ||||||
n, sample size; BMI, body mass index; Q1, lower quartile; Q3, upper quartile.
The results of multiple regression show that the BMI values have a larger share in the estimation of lumbar spine BMD variability than the age of women (Table 3). BMI, along with age, explained about 9.9-14.2% of the total BMD variability (according to adjusted R2).
The one-way analysis of variance to assess the variability of age- and BMI-independent measures of BMD (resL1, resL2, etc.) in the seasons of the women’s birth indicated a statistically significant relationship - for the first lumbar vertebra (resL1: F=2.37; p=0.043). Women born in summer have a lower resL1 as compared to those born in autumn (according to Bonferroni post-hoc test: p=0.046) – Table 4, Fig. 1. For the remaining 9 measurements of the lumbar spine, the observations of the lowest resBMD values in women born during summer do not exceed the threshold of statistical significance (p>0.05) – Fig. 2–3.
| BMD (g/cm2) |
Independent variables |
B | SE | t | p value | partial corr. |
Adj. R2 |
F | p value |
| L1 | Age (years) | -0.225 | 0.037 | -6.18 | <0.001 | -0.235 | 0.132 | 50.55 | <0.001 |
| BMI (kg/m2) | 0.297 | 0.037 | 8.15 | <0.001 | 0.304 | ||||
| L2 | Age (years) | -0.231 | 0.036 | -6.35 | <0.001 | -0.242 | 0.136 | 52.21 | <0.001 |
| BMI (kg/m2) | 0.300 | 0.036 | 8.22 | <0.001 | 0.307 | ||||
| L3 | Age (years) | -0.200 | 0.037 | -5.42 | <0.001 | -0.208 | 0.115 | 43.23 | <0.001 |
| BMI (kg/m2) | 0.285 | 0.037 | 7.74 | <0.001 | 0.291 | ||||
| L4 | Age (years) | -0.158 | 0.037 | -4.25 | <0.001 | -0.164 | 0.099 | 36.83 | <0.001 |
| BMI (kg/m2) | 0.283 | 0.037 | 7.60 | <0.001 | 0.286 | ||||
| L1-L2 | Age (years) | -0.235 | 0.036 | -6.48 | <0.001 | -0.246 | 0.142 | 55.00 | <0.001 |
| BMI (kg/m2) | 0.307 | 0.036 | 8.47 | <0.001 | 0.315 | ||||
| L1-L3 | Age (years) | -0.226 | 0.036 | -6.22 | <0.001 | -0.237 | 0.137 | 52.67 | <0.001 |
| BMI (kg/m2) | 0.305 | 0.036 | 8.38 | <0.001 | 0.312 | ||||
| L1-L4 | Age (years) | -0.209 | 0.037 | -5.72 | <0.001 | -0.219 | 0.131 | 50.15 | <0.001 |
| BMI (kg/m2) | 0.308 | 0.037 | 8.42 | <0.001 | 0.314 | ||||
| L2-L3 | Age (years) | -0.219 | 0.037 | -6.00 | <0.001 | -0.229 | 0.130 | 49.54 | <0.001 |
| BMI (kg/m2) | 0.298 | 0.037 | 8.15 | <0.001 | 0.305 | ||||
| L2-L4 | Age (years) | -0.200 | 0.037 | -5.47 | <0.001 | -0.210 | 0.124 | 47.21 | <0.001 |
| BMI (kg/m2) | 0.301 | 0.037 | 8.22 | <0.001 | 0.307 | ||||
| L3-L4 | Age (years) | -0.182 | 0.037 | -4.93 | <0.001 | -0.190 | 0.113 | 42.53 | <0.001 |
| BMI (kg/m2) | 0.294 | 0.037 | 7.96 | <0.001 | 0.298 | ||||
BMD, bone mineral density; BMI, body mass index; Beta, standardized regression coefficient; SE, standard error of standardized regression coefficient; t, t-Student test value; R2, coefficient of determination; F and p value for model.
|
Dependent variables |
Season of birth | F | p value | |||||||
| Spring | Summer | Autumn | Winter | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| resL1 | 0.003 | 0.152 | -0.024 | 0.136 | 0.018 | 0.133 | 0.009 | 0.137 | 2.73 | 0.043* |
| resL2 | 0.007 | 0.161 | -0.023 | 0.155 | 0.014 | 0.140 | 0.006 | 0.150 | 1.98 | 0.115 |
| resL3 | 0.004 | 0.175 | -0.022 | 0.160 | 0.015 | 0.155 | 0.007 | 0.168 | 1.67 | 0.172 |
| resL4 | -0.004 | 0.189 | -0.020 | 0.173 | 0.016 | 0.185 | 0.014 | 0.192 | 1.39 | 0.246 |
| resL1-L2 | 0.005 | 0.152 | -0.023 | 0.140 | 0.016 | 0.132 | 0.007 | 0.139 | 2.47 | 0.061 |
| resL1-L3 | 0.005 | 0.157 | -0.023 | 0.144 | 0.015 | 0.137 | 0.008 | 0.146 | 2.21 | 0.085 |
| resL1-L4 | 0.002 | 0.161 | -0.022 | 0.145 | 0.016 | 0.145 | 0.010 | 0.155 | 1.98 | 0.116 |
| resL2-L3 | 0.002 | 0.169 | -0.021 | 0.153 | 0.015 | 0.153 | 0.010 | 0.164 | 1.90 | 0.129 |
| resL3-L4 | 0.000 | 0.177 | -0.021 | 0.158 | 0.016 | 0.165 | 0.011 | 0.175 | 1.73 | 0.160 |
SD, standard deviation; F and p value, one-way analysis of variance (ANOVA) for season of birth on dependent variables: age-and BMI-independent measures of bone mineral density (BMD) by regressing BMD on age and BMI.
* The Bonferroni post-hoc test: Spring = Summer (p=0.441); Spring = Autumn (p=1.000); Spring = Winter (p=1.000); Summer ≠ Autumn (p=0.046); Summer = Winter (p=0.251); Autumn = Winter (p=1.000).
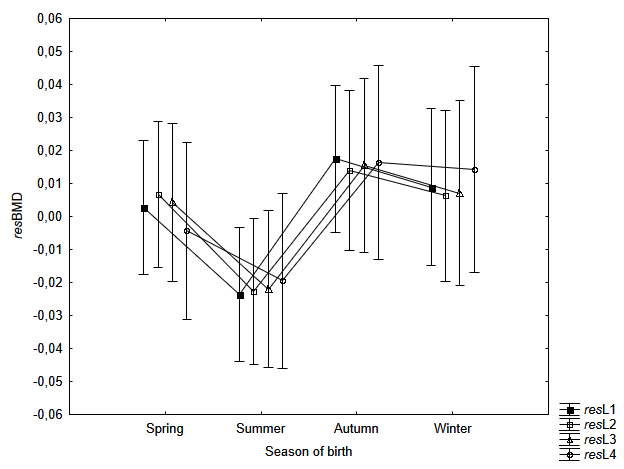
Fig. 1. Mean resBMD measurements of L1, L2, L3 and L4 according to the season of birth of examined women. Statistically significant differences in measurement of resL1 (F=2.37; p=0.043). According to the post-hoc Bonferroni test: significant difference (p=0.046) between women born in Spring (March-May) vs Autumn (September-November). Vertical bars indicate 0.95 confidence intervals
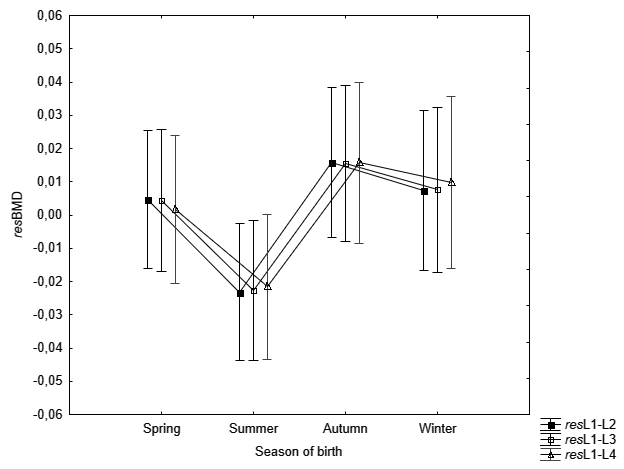
Fig. 2. Mean resBMD measurements of L1-L2, L1-L3 and L1-L4 according to the season of birth of examined women. Vertical bars indicate 0.95 confidence intervals
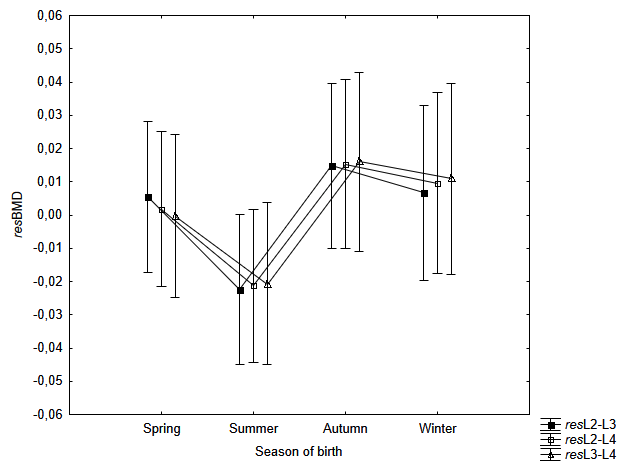
Fig. 3. Mean resBMD measurements of L2-L3, L2-L4 and L3-L4 according to the season for birth of examined women. Vertical bars indicate 0.95 confidence intervals
Discussion
The main result of the study is the indication of the significant relationship mineral density of lumbar vertebrae with the season of birth of women in the perimenopausal age. Women born in summer (June-August) were characterised by the lowest bone mineral density in the first vertebrae of the lumbar spine compared to women born in the autumn months (September-November). And this relation concerns the part of the BMD variability which is independent of age and BMI of the examined women.
The birth season is a direct consequence of the season of prenatal development, an environment, which is a multi-factor modifier of the human development during the first (most important) months of life. With regards to full-term children (gestation of 37–42 weeks), born in summer months (i.e., from the 1st of June through the 31st of August), the first two trimesters of gestation occur in autumn and winter months. Regarding the Central Europe, this part of the year is characterised by the lowest sun activity (insolation) (for the territory of Poland, the mean insolation level, measured by the number of sunny hours per month, is the highest from May through August – 243.17 and the lowest from November through February – 50.42). The autumn and winter months in Poland are also characterised by the lowest air temperature (the mean temperature level in summer months varies between 16.5 and 20°C, while it is only -6 to 0°C in winter), a limited availability of fresh vegetables and fruits, and a high incidence of infections (in Europe, the seasonal peak of influenza infections is usually between January and March). The insolation level significantly determines the synthesis of vitamin D which is delivered to the foetus exclusively from the mother’s body (Salle et al. 2000). Vitamin D deficits in pregnant women are significantly more frequently observed during winter months and in countries, where food stuffs are not routinely supplemented with vitamin D, also in members of the ethnic groups, in which women cover their bodies regardless of the season of the year and among persons with high pigmentation level of their skin (Specker 2004). Insufficient vitamin D levels in mother’s body compromise skeletal structure development and strength in the foetus, including, among others, lower bone mineral density, observed already in newborns (Tobias et al. 2005; Javaid et al. 2006; Cooper et al. 2009).
Maternal factors, which form the environment of prenatal development, “programming” the earliest stages of human skeleton formation, play a significant role in the epidemiology of osteoporosis (Cooper et al. 2009). These factors may include maternal nutrition (particularly deficient in vitamin D), maternal smoking and/or alcohol consumption during pregnancy. Low birth weight and body length as well as a low placental weight (an effect of vitamin D deficits in the prenatal environment) significantly correlate, both with low neonatal bone mineral (Dennison et al. 2001) and low neonatal bone mineral content (Godfrey et al. 2001). Reduced neonatal bone mass density leads to decreased adult bone mass density and, in consequence, to osteoporosis and an increased risk of hip fracture (Abrahamsen et al. 2012). Studies based on databases for European (UK and the Netherlands), US, Asian (Japan, Korea) and New Zealand population as well as the studies based on meta-analysis confirm this significant, one-way relationship of birth weight with lumbar bone mineral content (BMC) (Namgung et al. 1998; Baird et al. 2011). For example, a meta-analysis demonstrated that a 1000 g increase in birth weight was associated with a 1.49 g increase in lumbar spine BMC (95% CI 0.77-2.21) (Baird et al. 2011).
The season of birth is a significant newborn’s body weight regulatory factor. In European populations, (e.g., those in the Northern Ireland, Greece, Poland) the peak in childbirths with low body weight is recorded in spring and summer months (Murray et al. 2000; Flouris et al. 2009; Siniarska & Kozieł 2010). According to Murray et al. (2000), Irish children, born in July, have, on the average, lower (by 31.6 g) birth weight compared to children born in January (95% CI 35.2, 28.0). Polish children, born in April and May, exhibit the lowest average birth (Siniarska & Kozieł 2010).
Seasonal variation in maternal serum vitamin D levels is among the major causes of the observed relationship between birth season and biological features of man, including BMD and BMC, indicated by most researchers. In European populations, especially those inhabiting the Northern part of the continent, vitamin D synthesis is limited to 5-6 months during the year (Brot et al. 2001). This limitation, while determining the maternal vitamin D level, may significantly control vitamin D levels in child’s body, depending on the season of the year, during which prenatal development took place (characterised – at a given altitude – by specific conditions of exposure to sunlight, air temperature, the availability of fresh vegetable nutrition and the incidence of infections). The link between maternal vitamin D status and child bone mineral density was observed by Javaid et al. (2006), who measured BMD by DXA in 198 9-year-old children whose mothers had their serum 25 OH-vitamin D levels measured in the last weeks of the third trimester of pregnancy. Children of mothers with low vitamin D levels were characterised by much lower BMD values, measured both at the spine and in total body, as well as by lower levels of calcium ions in umbilical blood.
The prenatal and neonatal „programming” of disorders in mineral density of skeletal bones and, in consequence, of osteoporotic changes, is, in part, underlain by an epigenetic mechanism (Holroyd et al. 2012). Modification of PMCA3 (placental calcium transporter) gene expression level, which determines the neonatal whole-body BMC (Martin et al. 2007), may be of key significance in the control of vitamin D transport by the placenta, which, in turn, controls the prenatal level of ionized calcium concentration and, eventually, regulates skeletal growth and mineralization (Javaid et al. 2006).
The prenatal environment conditions, in which spine mineralisation processes take place, are thus of key importance in human BMC formation. It is possible that the prenatal environment conditions affect the peak bone mass attained at the age of 18 years, the low values of which are among the major risk factors of osteoporosis (Roy et al. 2005) and, in consequence, determine the rate of involutionary changes in the skeleton. According to Noback and Robertson (1951), the ossification of the spine spreads from two basic regions: the cervical and the lower thoracic/upper lumbar regions. Ossification in the lower spine region begins simultaneously at 3 points: within L11 and L12 vertebrae of thoracic spine and L1 vertebra of the lumbar spine. Only in further sequence do ossification centres occur in other vertebrae as well, both in cephalic and caudal directions (Bagnall et al. 1977). The ossification process in L1 begins on the 9th week of gestation and attains the L5 level at the end of the 3rd month. According to Scheuer and Black (Scheuer & Black 2000) the lumbar vertebrae are readily identifiable from the end of the fourth foetal month.
It is possible that the strength of the prenatal conditions depends on sex. According to Siniarska and Kozieł (2010), for instance, the influence of birth season on body length of the Polish newborns is characterised by distinctive “sex dimorphism”. In boys, the highest correlation between the average values of atmospheric characteristics and the neonatal body length was observed for the second trimester of prenatal growth, whereas in girls the highest correlation occurred for the first trimester.
The occurrence of ossification centres in L1 during the 2nd month of foetal life and, in subsequent lumbar spine vertebrae by the end of the 3rd month of foetal life, only begins the entire process of ossification. Fusion of the primary centres of ossification in the lumbar spine begins from L1 on the 1st year of postnatal life and continues in distal direction, attaining L5 at, approximately, the 5th year of child’s life (Scheuer & Black 2000).
It is therefore possible that the critical period for L1 vertebra development in children born in summer season (thus beginning the 2nd trimester of prenatal development in winter months, of the lowest exposure to sun light) is slightly more sensitive to the limitation of vitamin D levels and normal transport of calcium ions, thus being most susceptible to the consequential BMD reduction. According to Fønnebø (1995) and Abrahamsen et al. (2012), a low sunlight exposure prior to the crucial period in skeletal development should be considered as a risk factor of hip fracture in Northern European populations.
In conclusion, a significant diversity of bone density can be observed with respect to both the season of birth of the Polish women, and thus with respect to the season in which their prenatal development occurred. The analysis of variability of age- and BMI-independent measures of BMD (resL1, resL2, etc.) allowed us to indicate a statistically significant relationship for the first lumbar vertebra. Women born in summer have a lower BMD of the L1 vertebra compared to those born in autumn, regardless of the rate of bone density loss with age and a positive correlation with BMI.
The obtained results indicate the need to extend the group of risk factors for osteoporosis with the season of woman’s birth. The results of this study also suggest that women in Central Europe similarly and countries of the Northern Europe should be encompassed by special prophylactic care against osteoporosis of pregnant women, especially if the term of delivery is planned for summer months.
Acknowledgments
The authors would like to thank Katarzyna Plutecka who took part in collecting data and designing the database. The authors confirm that neither the manuscript nor any parts of its content are currently under consideration or published in another journal. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Conflict of interests
Authors declare no conflict of interests.
Authors’ contributions
EŻ and IR analyzed the data and drafted the manuscript. AES and MS collected the data. EŻ, IR and ES edited the manuscript for intellectual content and provided critical comments on the manuscript.
* Corresponding author: Iwona Rosset, Department of Anthropology, Faculty of Biology and Environmental Protection, University of Lodz, 12/16 Banacha St., 90-237 Łódź, Poland, tel.: +48 42 6354455, fax: +48 42 6354413, e-mail: iwona.rosset@biol.uni.lodz.pl
 https://orcid.org/0000-0003-1523-1912
https://orcid.org/0000-0003-1523-1912

