Introduction
Within the last three decades, breastfeeding and infant feeding strategies have become a one of the most important fields in anthropology, archaeology, evolutionary biology, and historical demography (e.g., Burt 2013; Britton et al. 2015; Tsutaya and Yoneda 2015; Cienkosz-Stepańczak et al. 2017; Crowder et al. 2019; Miller et al. 2020; Stantis et al. 2020; Loponte and Mazza 2021; Tomczyk et al. 2021; Chinique de Armas et al. 2022). Based on the connection between breastfeeding and signs of skeletal stress, weaning is assumed to exert an impact on individuals’ health later in their lives as well as population dynamics (King et al. 2018). This type of studies allows us to verify the hypotheses on the effect of parental investment (Kwok et al. 2018) and the structure of a group or family (Scharlotta et al. 2018).
Studying breastfeeding and weaning practices (BWPs) helps us understand how varied breastfeeding strategies were over the centuries and by how many factors (both biological and cultural) they were shaped. Cultural factors which are held to be the most critical socio-economic determinants of breastfeeding, supplementation or weaning practices include religious and social beliefs, type of economy and related food strategies described as subsistence economy (WHO 1998; Dettwyler 2004; Tsutaya and Yoneda 2015; Chinique de Armas and Pestle 2018). The last of the aforementioned factors is of particular interest to bioarchaeologists. Our research helped us determine that human groups dominated by specialised craftsmen, and traders were ones in which children were fed with mother’s milk longer and that breastfed children’s tombs were better equipped (Scharlotta et al. 2018). For example, the weaning process started earlier in hunter-fisher-gatherer groups than in agricultural and horticultural populations (Chinique de Armas and Pestle 2018). However, variations in breastfeeding duration are also observed within agricultural communities, which may stem from the type/species of plants grown and the source of protein consumed by the group (marine versus terrestial protein) (Chinique de Armas and Pestle 2018). It appears that the variability concerns not only the duration of the breastfeeding period but also the type of food fed to children: variation in diets immediately post-weaning, with increasing homogeneity in diet thereafter (Eerkens 2018). In this context, the availability of cultigens (for example zea mays) is not insignificant (Chinique de Armas and Pestle 2018; Schurr 2018). Going forward, factors influencing BWPs are also traced in studies involving diachronic comparisons of weaning age in the same populations. These studies demonstrate that reformation changes and growing urban environments may lead to changes in breastfeeding strategies (Britton et al. 2018). Moreover, research in context of the length of mother’s milk feeding includes cases of children buried in unusual ways. Researchers highlight that funeral rituals did not always result from the young age of the individual at the time of death, but it would be related to the loss of mother during birth or the first few days after birth (Craig-Atkins et al. 2018).
In conclusion, assessment of diet provides a valuable supplement to studies on origin and mobility. Of interest is the analysis of dietary practices pertaining to children after birth, including breastfeeding and weaning process. This subject is seen as important from the perspective of a child’s physical and psychological development, parental investment in offspring, and a reproductive strategies of a population (Lee 1996; Tsutaya and Yoneda 2015). Therefore, findings concerning breast-fed infants in archaeological populations may improve our current knowledge, not only in the cultural attitudes towards nutritional choices, but also women’s and children’s health, birth spacing, and the social and demographic structure of the analysed population.
Our knowledge about the diet of adults and children has significantly expanded thanks to the research on stable isotopes of carbon, nitrogen and oxygen. Stable isotope ratios of nitrogen and carbon in bone and dentine collagen have been used for over three decades to estimate palaeodiet, life strategies or breastfeeding duration of our ancestors (Szostek 2009; Arnay-De-La-Rosa et al. 2010; Schwarcz and Schoeninger 2012; McClure et al. 2020; Tomczyk et al. 2020a; Tomczyk et al. 2020b; Tomczyk et al. 2021).
In the studies of BWPs the most commonly used are nitrogen isotopes which reflect the general trophic level of consumed foods (Schoeninger 1985; Britton et al. 2018). Research performed on the hair of new-born infants and their mothers revealed no significant differences in isotope delta between the woman and her child (Fuller et al. 2006; De Luca et al. 2012; Dailey-Chwalibóg et al. 2020). In infants breast-fed over the first weeks of life, δ15N values in hair keratin rise until a 2–3‰ difference is reached, relative to the value of the parameter in their mother’s hair (Fogel et al. 1989). This difference in isotope proportions maintained until the first non-maternal food is introduced in the child’s diet. When weaning begins, the child starts consuming protein sources other than mother’s milk, the isotope delta of nitrogen gradually decreases and eventually fall in line with δ15N of the mother and other adults from that population (Katzenberg 1996; Lewis 2007). The improvement of the method is the Weaning Age Reconstruction with Nitrogen isotope analysis (WARN) model for analyzing cross-sectional δ15N data of subadult bone collagen, which is used for reconstructing precise weaning ages (Tsutaya and Yoneda 2013).
Longitudinal analysis of stable carbon isotopes in the keratin of hair and nails of infants and their mothers show that during breastfeeding, keratin in infants is enriched with heavy carbon isotopes (13C) and that the average difference between the offspring and their mother is c.a. 1‰ (Fuller et al. 2006). In other studies of proteins isolated from hair, the carbon isotope ratio differed between mothers and newborn infants by 0.9 ‰, and in the case of nitrogen the difference was 0.4 ‰ (De Luca et al. 2012). The process of carbon incorporation into bone occurs in balance with carbonates bound in the blood and such carbonates are products of nutrient metabolism. Authors concluded that δ13C analysis allowed them to retrace the moment when solid food, with different isotopic proportions from mother’s milk, was introduced into the diet, and what could be the proportion of C3 and C4 plants in the diet (e.g., Eerkens et al. 2018) whereas δ15N values were the record of the duration of milk consumption (Fogel et al. 1989; Fuller et al. 2006; Tsutaya and Yoneda 2015; Cienkosz-Stepanczak et al. 2017; Stantis et al. 2020). In all of the cited studies, the isotope values of breastfed infants were higher than those of the mothers. However, despite the hair tests set a certain model of changes in isotope values between mother and offspring, it should be emphasized that short-term changes in δ15N hair values may not reflect dietary shifts, but may be the result of the changes in an individual’s metabolic balance owing to specific physiological events (D’Ortenzio et al. 2015).
Isotope analysis of the weaning process typically involves the comparison of isotope ratios in the tissues of children weaned at different ages and isotope levels in women of reproductive age. The start of the weaning process is defined as the moment in which isotope levels in the child drop and approaches those of the isotope levels in the mother. The weaning age is the moment when the isotope delta of those two groups equalize (Dupras & Tocheri 2007; Jay et al. 2008; Choy and Richards 2009). This approach was improved through the application of mathematical models proposed by Schurr (1997) and Tsutaya and Yoneda (2013). Nevertheless, it should be noted that the relationship between adult/child diet and carbon and nitrogen isotope delta has yet to be fully explored. C and N isotope ratios in collagen may be affected by a specific fragment of the skeleton (bone/tooth type and its section) characterised by different bone turnover and remineralisation rate, and even short-lived individual physiological changes during bone growth, pregnancy, and breastfeeding, and, importantly, nutritional, and physiological stress, as well as diseases caused by diet modifications (Katzenberg 1999; Lewis 2007; Beaumont et al. 2015; Britton et al. 2018; Beaumont 2020). Interpretation of C and N isotope analysis results may also be hindered if children experienced non-standard breastfeeding or weaning practices (Tsutaya and Yoneda 2015).
Currently, isotopic proportions taken from microscopic enamel or dentine incremental layers is considered to be the most precise research tool for capturing the most likely moment of a change in the diet of a child from exclusive breastfeeding to complete weaning (Humphrey et al. 2004; Fuller et al. 2003 as cited in Lewis 2007). However, all methods have limitations, in this case there are also a few to mention.
In addition to analyses of carbon and nitrogen isotopes derived from collagen, which is more susceptible to diagenetic processes than enamel, analysis of the weaning process may also be performed using a method based on stable oxygen isotopes in a mineral fractions of enamel and dentin (Montgomery 2010; Budd et al. 2000; Dudás et al. 2016; Simpson et al. 2021). Wright and Schwarcz (1998; 1999) made an attempt at using apatite from the teeth of analysed individuals, which currently seems the optimum solution to the issue of reconstructing the weaning age based on skeletal minerals. This solution uses knowledge of the duration and sequence of mineralization in each tissue (enamel, dentine) for various types of teeth. In practice, researchers compare the isotope composition of enamel or dentine for various types of teeth in an individual (Wright and Schwarcz 1998; Wright and Schwarcz 1999; White et al. 2004b) or isotope levels in a child’s teeth with corresponding values obtained for the teeth of women of reproductive age (White et al. 2004b). However, in many studies it is impossible to carry out the reconstruction of weaning based on odontological material because of limitations such as the absence of odontological material in the uncovered skeleton, insufficient amount of teeth to conduct isotope analyses, the presence of significant carious lesions, or no possibility of sacrificing teeth for isotope analysis (irreversible damage to the tooth’s structure in the preparation procedure).
With time, around 2000, weaning studies started analysing relationships between nitrogen and oxygen isotopes. It is assumed that stable isotopes of both elements reflect changes in breastfeeding but involve different physiological mechanisms (Britton et al. 2018). While nitrogen isotope methodology is based on protein metabolism, oxygen isotope ratios are indicative of isotope fractionation in body water. Body water is characterised by a higher oxygen isotope level than environmental water due to fractionation in metabolic processes (Luz et al. 1985; Bryant and Froelich 1995). On the basis of previous studies on archaeological dental material, it may be concluded that children not fed with mother’s milk have lower isotope proportions compared to breastfed children, similarly to weaned children, because after weaning, when their bodies start absorbing water from food (oxygen source) rather than water contained in milk, there should be a drop in their δ18O level (Wright and Schwarcz 1999; Lewis 2007).
Stable oxygen isotope analyses were first applied in research on the paleoclimate (Cormie et al. 1994; Iacumin et al. 1996; Tütken et al. 2006) and are currently used in bioarchaeology to investigate multiple aspects of life in ancient populations (e.g., Evans et al. 2006; Lee-Thorp and Sponheimer 2006; Knudson and Torres-Rouff 2009; Price et al. 2010, 2019; Perry et al. 2020). Reconstruction of the place of origin and movement of populations may help retrace the dynamics of mobility, possible exchange of commodities, contacts, and cultural diffusion of human groups. Isotope analyses are commonly conducted in the context of origin and migration and concern an increasing number of diverse archaeological sites (e.g., Dupras and Schwarcz 2001; Depaermentier et al. 2020; Leach et al. 2010; Price et al. 2010; Lisowska-Gaczorek et al. 2016; Harris et at. 2017; Osipowicz et al. 2017; Turner 2021).
Isotopic approaches such as bone phosphate oxygen may be useful in confirmation and further characterizing trends observed in an analysis of bulk bone collagen or/and enamel/dentin incremental layers. This subject requires further analysis, and the potential for application appears significant in certain cases, e.g., if collagen is poorly preserved and has undergone extensive diagenesis. Bone mineral, particularly enamel, is the tissue most resistant to diagenetic processes (Montgomery 2010; Cienkosz-Stepańczak et al. 2021).
The selection of research method/technique depends on the availability of the material and the condition in which it is preserved. When collecting samples from bones, areas displaying signs of lesions or fractures are omitted. In addition, an analysis of diagenesis is performed. The condition of odontological material is assessed for the extent of diagenetic alterations, the presence of fractures and noticeable intra vitam tooth degradation (crown wear, dental caries). In the context of diagenesis, of high significance are tissue properties such as collagen content, histological integrity, porosity (water absorption potential), crystallinity (Hedges 2002), and on the other hand, the presence and extent of the effect of specific chemical factors in the grave environment, exerting a degrading influence on skeletal material (diagenetic factors) (Kendall et al. 2018).
In general, dental tissues are better preserved post mortem due to the resistance of enamel and a less porous structure than in bones. However, certain exceptions still apply (Hollund et al. 2015).
Similarly to collagen, bone hydroxyapatite is exposed to diagenetic factors but less susceptible to its effects. One of the reasons for its increased resistance to diagenesis is the fact that bone mineral is protected in the initial stages of decay by collagen. A substantial collagen loss in bones is correlated with microbial activity (Hedges 2002). It should be emphasised that tooth enamel has larger, neatly ordered bioapatite crystallites, which contain less carbonate and more fluorine than bones (Wopenka and Pasteris 2005). In addition, enamel has lower organic content (<1% by volume), as opposed to bone (32–44% by volume) (Olszta et al. 2007), and the presence of organic compounds plays a key role in diagenesis.
For teeth, larger crystallites, lower carbonate proportions, low collagen content, and the presence of F ions in in vivo enamel results in a more thermodynamically resistant material in comparison to bone (e.g., Wopenka and Pasteris 2005). In the event of insufficient odontological material and/or diagenesis of a part of the dental tissue, the solution is to choose the bone component which is more stable in the harsh environment of the tomb. In the light of the above juxtaposition of tooth and bone tissues, we decided that phosphates rather than collagen should be the subject of our study.
Oxygen isotopes in bone and tooth apatites as a source of information on the weaning process
Due to the isotope fractionation process, mother’s body water and produced milk may contain increased oxygen isotope ratios compared to local water drunk by the mother (Wright and Schwarcz 1998). The research by Kornexl et al. (1997) shows that the oxygen isotope of cow’s milk was on average about 2–6 ‰ higher compared to drinking water. The experiment of Lin et al. (2003) showed that cow’s milk was on average about 4‰ heavier isotope than water, while in analogous studies by Camin and colleagues (2008) found a similar regularity of about 2 ‰. The child’s total water intake includes water from the oxidation of human milk proteins, lipids and carbohydrates, and water from sources other than human milk. Exclusive breastfeeding means that the infant receives human milk without any additional food or drink, not even water. For an exclusively breastfed infant, the mother’s milk is the main source of oxygen (the proportion of oxygen in the bone tissue from the atmospheric air is insignificant). Due to the fractionation between the oxygen contained in milk and body water, the breastfed offspring builds into its tissues a pool of oxygen, the isotope composition of which is potentially even more enriched in heavy isotopes of oxygen 18O (Figure 1) (Wright and Schwarcz 1998). We may expect that tissues mineralized during this period (bones or teeth) will display this kind of enrichment in heavier isotopes (18O) until the start of the weaning process. If mother’s milk is excluded from the diet, local drinking water becomes the main source of oxygen and δ18O level in tissues of a young individual should drop to the level of the mother’s isotope delta (Figure 1). It should be noted that after weaning, the isotopic values of nitrogen and carbon in a child may differ from that of the mother due to eating foods with higher or lower trophic levels, however, after weaning, the children were fed only porridges and plant food, for example, in other populations animal milk (e.g., Dupras et al. 2001). Weaning studies using hydrogen isotopes show that both the cooking water and the partially-soluble barley fraction become elevated in 2H as a result of cooking (Ryan et al. 2020). It seems that in the case of oxygen isotopes, the isotopic proportions in sceletel tissues of children may also differ after complete weaning from mother’s milk, for example, due to thermal processing of food or consumption of animal milk.
However, it should be emphasized that despite the noticeable tendency for oxygen isotope ratios to increase in the teeth of individuals who were probably breast-fed, the exact course of this phenomenon could not be retraced, since it is not possible to perform relevant research on living individuals (the necessity to collect bone fragments and teeth from an individual at several stages of ontogenetic development). Researchers acknowledged the problem and it was presumably for this reason that relatively few of them decided to study breastfeeding practices and determine the weaning age using stable oxygen isotopes, although stressing that such an approach has considerable potential (Britton et al. 2015).
Research using tissues collected from animals in a controlled experiment could be a solution to this problem. This type of experimental study allows us to avoid the so-called “osteological paradox”, as well as additional variation resulting from the introduction of water with diverse isotope content into the body or the effect of taphonomic factors on the skeleton or the effect of previous illnesses on isotope levels measured in bones.
Another important aspect of oxygen isotope analysis that may pertain to reconstruction of the breastfeeding and weaning process is the influence of culinary practices on the isotope composition of beverages and liquid food on mineralizing bone tissue. Isotope ratios in consumed beverages may diverge from “local” ones due to boiling, brewing, or stewing of meals, which has only recently been explored in experimental studies (Brettell et al. 2012; Royer et al. 2017; Lisowska-Gaczorek et al. 2020). The literature emphasizes that culinary practices involving thermal treatment of water may lead to a substantial depletion of the isotope pool of 16O in body water, which is reflected in isotope ratios of bone tissue (Daux et al. 2008; Brettell et al. 2012) and increased oxygen isotope ratios in the body. Apparently, drinking water that has been thermally processed for an extended period (2.5 h) increases its isotope value by 6.1 ‰; an increase in isotope delta of phosphatic apatites by approx. 4 ‰ was reported in animals drinking isotopically enriched water (Lisowska-Gaczorek et al. 2020).
To date, however, it has not been investigated whether drinking mother’s milk (a source of oxygen enriched by isotope fractionation in the metabolic process of mother’s milk production) influences oxygen isotope levels in bone tissue of offspring, and consequently, the interpretation of results of such studies.
Considering that the effect of changes in oxygen isotope level in bones related to a change in the trophic level and during weaning has not been investigated, this study is an attempt to evaluate the influence of the replacement of mother’s milk with another source of oxygen on the isotope ratios of weaned individuals.
The goal of this work was to ascertain whether thermal processing of potable water is related to a change in the oxygen isotope ratio in breast-fed and weaned individuals. Additionally, the isotopic effect of the change in trophic level of the boiled water model was analysed. It should be noted that due to the analysis of fresh teeth and bones, diagenesis does not affect the isotopic proportions obtained in this study and does not affect the interpretation in any way.
Our hypotheses concern primarily the isotope effect related to a change in the trophic level. We assume that isotope fractionation in a physiological process like milk production leads to a higher oxygen isotope level in the tissues of breastfed individuals than in their mother’s skeletons. Besides, weaning and the introduction of another source of oxygen isotopes to its diet means a change in the trophic level of the young organism. Our experiment, carried out on livestock in uniform conditions, may allow us to determine if and to what extent extra-dietary factors influence oxygen isotope variation.
Material and methods
Analyses were performed on an animal model of the Wistar Cmd:(WI)WU rat strain. Since rodents, such as rats and mice, are used in model studies on human metabolism, genetics, and physiology, including skeleton development and mineralization (Smith and Warshawsky 1975; Jheon et al. 2013), we decided to use this model in the present work. All procedures involving animals were approved by the Ethical Committee of The Jagiellonian University in Krakow, Poland (no. 122/2011) in accordance with international standards.
A total of 8 females, divided into two groups, were used in the experiment. The number of animals that were used in the experiment resulted from applying good practices in animal studies. The first group drank tap water from the water supply network, while the second group drank boiled water. Tap water was collected from the network over the period for which the rats were kept, i.e., less than 3 months to exclude seasonal variation in the isotope levels in environmental water. The method of preparing boiled water has been described in detail in the publication on the first part of the experiment (Lisowska-Gaczorek et al. 2020). Rats were kept at a stable humidity (55%), lighting (artificial light 60lx, 12 h/12 h), temperature (22°C), and with ad libitum availability of feed and water. The only differentiating factor was the type of consumed water. Pregnant females (8 rats), who were already drinking water appropriate for their group three weeks prior to mating, gave birth to offspring that were subsequently placed in cages for female rats and divided according to the type of water provided. The group of offspring of the mother drinking tap water included 19 rats, while the group of offspring of the mother drinking thermally processed water included 33 rats. All young offspring had access to mother’s milk until the 21st day of life, with a few of them fed in this way several days longer. After this period, mothers and half of the offspring from each cage were put down. Instead of milk, the remaining rats went on to drink the same type of water as that given to their mothers, until the 69th day after weaning. Afterwards, they were put down. Each mother was kept in a separate cage with its offspring, which enabled separate/individual analysis for each subgroup, with the exception of those subgroups in which the offspring did not survive.
Teeth and femurs were collected from each animal. The analysis took into account long bone apatite isotope ratios. Meanwhile, in a situation where isolating bone phosphates was not possible for technical reasons, we analysed teeth to supplement the material. Rat teeth mineralize and grow over the entire lifetime like bones, additionally high bone turnover increases the amount of tooth movement (Verna et al. 2003) therefore it was possible after checking isotope differences between the two types of tissue.
Material cleansed of soft tissues was dried, ground, and weighed into 0.4 g portions. Next, apatite phosphates were isolated from bones and teeth in accordance to the procedure designed by O’Neil and collaborators (1994) and as described by Vennemann (2002).
The isotope composition of oxygen in the extracted phosphates was determined at the Department of Radioisotopes, Institute of Physics-CSE, Silesian University of Technology, Gliwice, Poland using continuous-flow isotope ratio mass spectrometry. The results of isotope analysis are presented in delta notation δ18Osample=[(Rsample-Rstandard)/Rstandard]*1000; R=[18O]/[16O]. VSMOW was used as a reference for the NBS120C standard (Lécuyer et al. 1996). Pre-treatment of the NBS 120C samples was identical for all apatite samples. A NBS 120C standard sample was also analysed (δ18O=21.7‰).
The isotope ratio in rats is presented in delta notation (δ18O). In several places, the following markings were used: the subscript indicates the individual’s age class: “f” – females/mothers, “y” – young breastfed offspring, and “o” – older weaned offspring. For example, δ18Oo represents older offspring weaned off mother’s milk.
Comparisons of isotope results in groups differing in the type of analysed tissue, as well as the age and type of consumed water were carried out using the Mann-Whitney test. The normality of distributions was tested with the Kolmogorov-Smirnov test. We assumed the significance level of p < 0.05.
Application of the experiment in bioarchaeological studies
One of the key outcomes of the present experiment, in addition to the isotope effect observed in the form of the change in the trophic level, was an attempt to study the dynamics of changes in 18O level as a result of weaning in the human species. For this purpose, we employed regression equations describing the relationship between the isotope composition of drinking water and phosphates for rats (Luz and Kolodny 1985) and for humans (Daux et al. 2008; Equation 6).
In the tap water model, phosphate isotope values for rat females/mothers were converted to isotope levels for water (Equation A: δ18Op= 0.49xδ18Ow+ 17.88 designed by Luz and Kolodny 1985) to determine the common baseline value for organisms that potentially drank the same water. Equation B: δ18Ow= 1.54 (±0.09)x δ18Op – 33.72 (±1.51) was employed to estimate isotope levels which we expect to be characteristic of individuals who drank water of a similar isotope composition to the water ingested by rats (Daux et al. 2008; Equation 6). In this way we estimated the isotope ratio for mothers/women whilst being able to link the data to the experiment. First, in order to estimate isotope proportions in children who drank milk from the aforementioned mothers, we attempted to determine the approximate value of the isotope ratio for milk consumed by the offspring. Based on earlier findings, according to which body fluids such as urine, milk, saliva, sweat, and blood have similar oxygen isotope ratios (Bryant and Froelich 1995), we applied a regression equation for the relationship between drinking water (δ18Odw) and body water (δ18Obw) isotope ratios – Equation A’: δ18Obw= 0,5x δ18Odw -0,68 ‰ (Valenzuela et al. 2021; Equation 3). Another assumption concerned young rats. Milk drank by rats (or, specifically, water in the milk) was the only potable source of oxygen. Hence, for small children, we assumed that δ18O for milk equals δ18O for drinking water. Then, as previously for women, we used Equation B (Luz and Kolodny 1985) to estimate the isotope ratio in children who consumed milk with the isotope proportion calculated in the previous step. In this way, we obtained estimated δ18Op values for breastfeeding mothers and children fed exclusively with milk.
Results
It was necessary to verify any statistically significant differences between isotope delta for oxygen collected rat tissues in each age class, considering the division into the tap water and boiled water group. The results of the Mann-Whitney test for each group indicated that the isotope composition of bones and teeth did not vary significantly in either group (Table 1).
Due to the absence of differences, when for a given animal it was not possible to isolate phosphates from bones but only from teeth, the results of isotope analyses were interpreted together, in order not to minimize the research group.
Oxygen isotope data provided the basis for subsequent stages of the presentation of results and are shown in Table 2.
| Age | tap water group | boiled water group | ||||
| N | p | N | p | |||
| bone samples | teeth samples | bone samples | teeth samples | |||
| Mothers | 4 | 3 | 0.38 | 3 | 3 | 0.19 |
| Breast-fed rats | 10 | 10 | 0.34 | 16 | 16 | 0.98 |
| Weaned rats | 6 | 9 | 0.22 | 12 | 10 | 0.34 |
since the p-value is greater than or equal to 0.05, there is not a statistically significant difference between the medians at the 95.0% confidence level.
| Age | Skeletal fragment | N | x | S | V [%] | Me | min | max | |
| Tap water | Females/ mothers* | bone | 4 | 13.0 | 0.1 | 1.1 | 13.1 | 12.8 | 13.1 |
| Breast-fed rats about 3 weeks | bone | 10 | 14.8 | 1.2 | 8.0 | 14.7 | 12.8 | 16.6 | |
| Weaned rats about 3 months* | bone/tooth | 9 | 14.2 | 1.0 | 7.0 | 14.2 | 12.6 | 16.0 | |
| Boiled water | Females/ mothers* | bone/tooth | 4 | 16.9 | 1.9 | 11.7 | 16.6 | 15.1 | 19.2 |
| Breast-fed rats about 3 weeks | bone/tooth | 20 | 18.4 | 1.1 | 5.9 | 18.4 | 16.0 | 19.9 | |
| Weaned rats about 3 months* | bone/tooth | 13 | 17.6 | 1.4 | 8.1 | 18.2 | 14.9 | 19.4 | |
where; n - numbers
x - mean average
Me - median
s - standard deviation
min - the lowest value in the range
max - the highest value in the range
V - coefficient of variation
* Lisowska-Gaczorek et al. 2020
The theoretical model of the isotopic effect related to a change in the trophic level suggests observable changes in the isotope ratios of biological apatite as a response to the introduction of a different oxygen source into the body (Figure 2). Because of this, the oxygen isotope variation for apatite phosphates in females/mothers (δ18Of), young offspring aged up to approx. 3 weeks (δ18Oy), and older offspring (δ18Oo) was traced. The analysis was performed separately for the tap water and boiled water model groups. The results are presented in Table 2 and Figure 1. In both models, females who drank water throughout the experiment had the lowest isotope values. Meanwhile, the highest isotope delta values were observed in individuals fed with mother’s milk and intermediate values for rats who were weaned and had not drunk mother’s milk for 69 days.
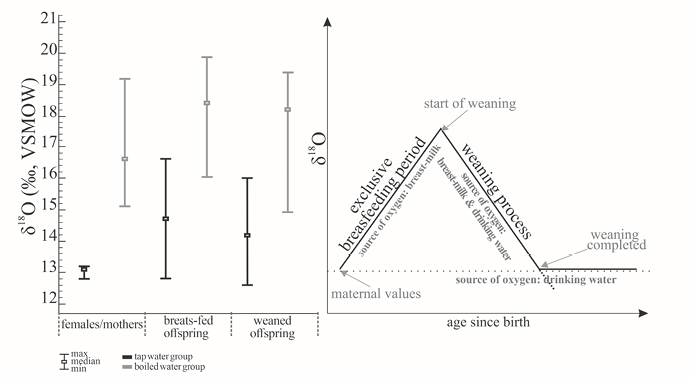
Fig. 1. Oxygen isotope diversity in apatite phosphates for mothers/breastfeeding females, children drinking mother’s milk and weaned individuals in the tap water model (black line), boiled water model (grey line) in juxtaposition with the theoretical diagram, in which the dotted line stands for the mother’s oxygen level and the solid line indicates the offspring’s changing oxygen isotope level
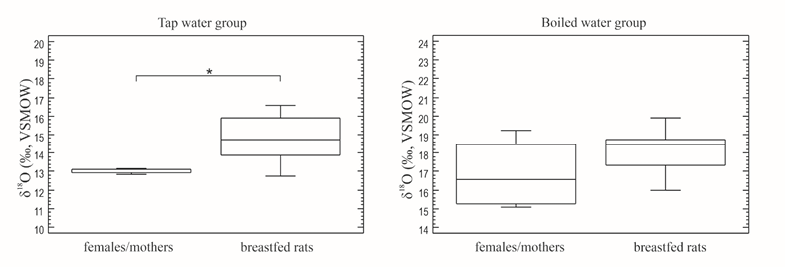
Fig. 2. Comparison of isotope values of mothers and milk-fed offspring by type of water consumed by female / mothers
To determine the effect of mother’s milk intake on the isotope ratio of bone apatite, isotope data obtained for mothers were juxtaposed with isotope ratios for breast-fed offspring. In the group of animals with access to tap water, the isotope ratio for breast-fed individuals was 1.6‰ higher than for their mothers, while the stable oxygen isotope ratio for breast-fed young rats in the boiled water model was 1.8‰ greater than the same parameter for their mothers (Table 2, Figures 1 and 2).
A comparison of isotope values for mothers and their breastfed offspring using the Mann–Whitney nonparametric test shows that the difference between the medians in the group in which mothers drank tap water, is statistically significant (W=36.0; p=0.0283), whereas there is no statistically significant difference in the boiled water group (W=56.0; p=0.2298) (Figure 2).
After 69 days, which passed from the moment in which the offspring was denied any access to mother’s milk, δ18O values in both tap water and boiled water models dropped. In some of the offspring the isotope level became equal to that of mothers, while in others the effect of weaning manifested itself more slowly (Figure 1). In the tap water model, oxygen isotope delta for weaned individuals dropped 0.5‰ in comparison to breast-fed ones (median). In the boiled water model, the reported decrease in the isotope ratio was less noticeable and equalled 0.2‰.
The next step involved testing the dispersion of δ18O values for females, offspring fed with their milk, and weaned offspring in each subgroup (e.g., female 1 vs offspring breastfed by female 1 vs completely weaned offspring of female 1). Table 3 and Figure 3 present an analysis of the variation in oxygen isotope proportions among the offspring of each female in the tap water model; Table 4 and Figure 4 show groups from the boiled water model. Groups from the tap water model are marked by the letter Z, while groups from the boiled water model are marked by the letter G.
Groups of breastfed young rats which were varied in terms of the type of water drank by their mothers had different isotope ratios. The 3.7‰ difference between median values for the two groups of breastfed rats (Table 2) referred to above was statistically significant, as confirmed by the results of the Mann-Whitney test (W= 6.0; p<0.001).
| N | breastfed vs females | weaned vs females | breastfed vs weaned | ||||||||
| breastfed | weaned | Me | min | max | Me | min | max | Me | min | max | |
| female Z1 | 2 | 3 | 1.7 | 1.6 | 1.7 | 1.3 | 1.3 | 1.6 | 0.4 | 0.3 | 0.1 |
| female Z3 | 4 | 4 | 2.5 | 0.7 | 2.9 | 1.7 | 1.1 | 2.8 | 0.8 | -0.3 | 0.1 |
| female Z4 | 4 | 2 | 1.2 | -0.4 | 3.4 | -0.3 | -0.5 | -0.1 | 1.5 | 0.2 | 3.5 |
Z – a particular group of rats: a mother drinking tap water and her offspring
1,3,4 – individual group numbers
| N | breastfed vs females | weaned vs females | breastfed vs weaned | ||||||||
| breastfed | weaned | Me | min | Max | Me | min | max | Me | min | max | |
| female G1 | 4 | 5 | 3.2 | 1.4 | 4.8 | 0.3 | 1.4 | 1.5 | 0.3 | 1.4 | 1.5 |
| female G2 | 4 | 4 | -0.3 | -0.1 | -3.2 | 2.2 | 1.1 | 0.7 | 2.2 | 1.1 | 0.7 |
| female G3 | 5 | 0 | 1.7 | 0.7 | 3.1 | - | - | - | - | - | - |
| female G4 | 5 | 4 | 0.9 | -0.2 | 1.6 | -0.2 | 0.7 | -0.1 | -0.2 | 0.7 | -0.1 |
G – a particular group of rats: a mother drinking boiled water and her offspring
1–4 – individual group numbers
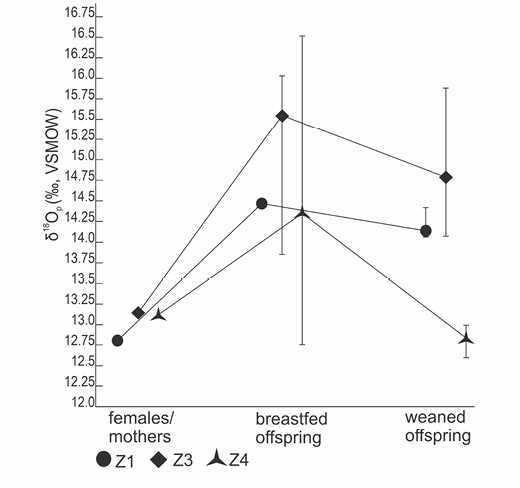
Fig. 3. Analysis of individual mother-offspring groups- tap water model. Z – a particular group of rats: a mother drinking tap water and her offspring; 1,3,4 – individual group numbers
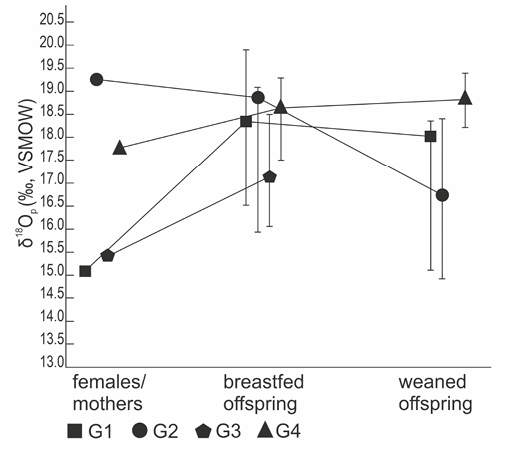
Fig. 4. Analysis of individual mother-offspring groups- boiled water model. G- a particular group of rats: a mother drinking boiled water and her offspring; 1–4 – individual group numbers
In addition, variation in the isotope effect caused by the change in the source of oxygen introduced in the rat’s body at different development stages/ages was observed in both models (Table 3, 4, Figure 3, 4). In the tap water model (Table 3, Figure 3), the greatest difference between the isotope ratio in the mother’s and her suckling’s bone tissue was reported for female Z3 (∆=2.5‰), and the smallest for female Z4 (∆=1.2‰). In contrast, a comparison between isotope ratios for breastfed and weaned rats indicates that in group Z4 (∆=1.5‰) the difference is the most prominent, whereas the smallest difference is present in group Z1 (∆=0.4‰).
In the boiled water model (Table 4, Figure 4), the most salient difference in δ18O for females and breastfed offspring was in group G1 (∆=3.2‰), while in group G2 oxygen isotope level in the apatite from breastfed rats was slightly lower than in their mothers (∆=-0.3‰). In G2, rats weaned off mother’s milk were the quickest to lower their isotope ratio post-weaning (∆=2.2‰) and for G4 the median for weaned rats was higher than one for breastfed individuals (∆=-0.2‰). Note, however, that in the latter group no weaned individual attained an apatite isotope value significantly higher than the highest level of δ18O reported for an individual breastfed by a mother from group G4.
Application of our models in bioarchaeological studies
Tap water
One of the aims of this study was to determine the way in which the results could be used in the context/interpretation of bioarchaeological research. In an attempt to establish a common denominator for humans and rats, the isotope ratio for apatite phosphates in female rats who had only drunk tap water (δ18Op=13.1‰) (Table 2) was calculated based on Equation A, designed by Luz and Kolodny (1985), as the δ18Ow value of potential drinking water. As a result, the value of -9.8‰ was obtained (Step I, Figure 5).
In order to verify what isotope ratio in bone apatites a human drinking water with a value of –9.8‰ would have, an equation proposed by Daux et al. (2008; Equation 6) was used, returning 15.6‰ as the result (Step II, Figure 5).
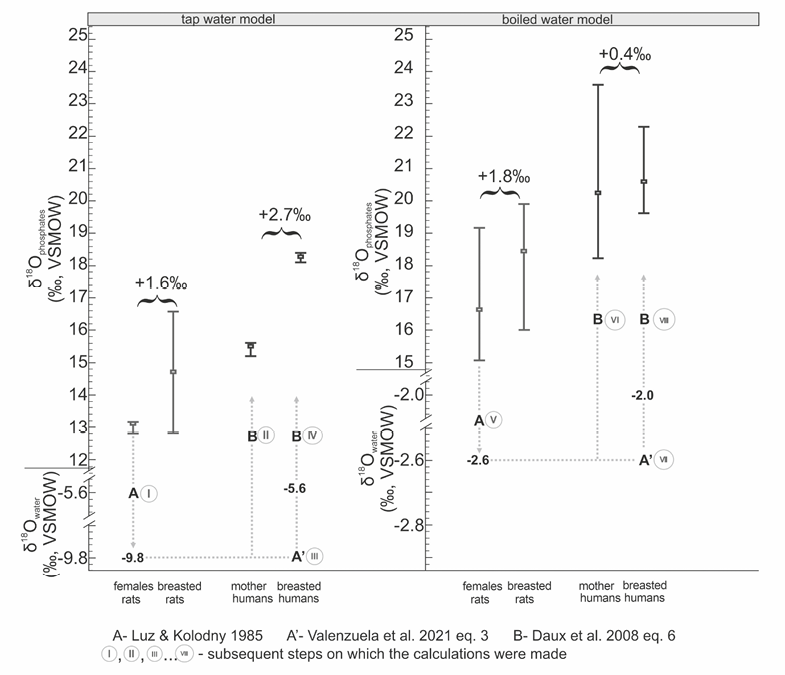
Fig. 5. Estimated differences in the isotope composition of oxygen in human bones, from mother to child, based on the results of the experiment and theoretical assumptions for the tap water and boiled water models. Roman numerals I–VIII were used to mark references to the text: steps according to which the conversions were made
A further step was to establish (approximate) values of oxygen isotope ratio for a human child breastfed by a mother who is assumed to ingest water with an oxygen isotope ratio of -9.8‰. For humans, there is a commonly applied regression equation describing the relationship between oxygen isotope ratios in drinking water and body water. Water’s isotope value (potential source of water for the population of the Krakow area) of -9.8‰ was converted to body water values (Step III, Figure 5). Assuming that mother’s milk contains isotopes in proportions similar to those of body water, whose δ18O level was -5.6‰, we employed Equation B to calculate the value for bone phosphates expected in children fed from an oxygen source having the aforementioned isotope proportion (Step IV, Figure 5). It was estimated that children breastfed with mother’s milk with an isotope value of approximately -5.6‰, resulting from the regression linking the drinking water isotope ratio (δ18Odw) and the body water isotope ratio (δ18Obw), could have an isotope value for bone phosphates equal to 18.3‰.
Boiled water
The same analysis was performed separately for the boiled water model (Figure 5). As in the model presented above, Equation A (Luz and Kolodny 1985) was used to verify the isotope ratio of the oxygen source ingested by females/mothers in the form of (boiled) water. The isotope ratio for water calculated by converting Equation A is -2.6‰ (Step V, Figure 5). Applying an equation developed by Daux et al. (2008; Equation 6) (Step VI, Figure 5), we were able to estimate the isotope ratio for bone phosphates in a (human) mother at 20.2‰, i.e., 4.6‰ higher than in a woman drinking water without any thermal processing. The value for female body water (and, by extension, also for milk) (Step VII, Figure 5) was calculated as approximately -2‰, whereas the isotope ratio for bone phosphates in breastfed children was 20.6‰ (Step VIII).
As shown in Table 2 and Figure 5, the difference between mothers and offspring in the case of tap water equalled 1.6‰, while for boiled water it was 1.8‰. For the estimated human ratios, δ18O level in breast-fed children can be enriched by 2.7‰ and if their mothers ate long-boiled meals and drank long-boiled beverages, the difference was smaller and amounted to 0.4‰ (Figure 5).
Discussion
Biochemical tests on skeletal material are increasingly often applied in bioarchaeology since they may provide plenty of valuable knowledge on the lives of our ancestors. Yet, such tests are beset with certain limitations due to poorly preserved biogenic structure of the material as well as incomplete knowledge of the phenomena determining the quantitative and qualitative incorporation of dietary components into bone structure.
Providing a full explanation of many of those phenomena would require a long-term observation of isotope ratios in the bone tissue in contemporary individuals, taking into account multiple internal and external factors which would potentially affect those values. For this reason, based on biological knowledge, researchers take advantage of various model studies, including ones based on experimental research (Fogel et al. 1989; Fuller et al. 2003; IAEA 2010; Brettel et al. 2012; Herrscher et al. 2017; Tuross et al. 2017; Leichliter et al. 2021). One of them involves the creation of an animal model (e.g., Leichliter et al. 2021), as in the present study. As already mentioned, rats are very frequently used as model animals in studying multiple aspects of the functioning of the human body (Smith and Warshawsky 1975; Jheon et al. 2013).
In the first part of the analysis of the results it was found that the oxygen isotope composition for teeth and bones in the rat groups did not differ in a statistically significant way (Table 1), which is probably due to the fact that rat teeth are elodontic, i.e., they grow continually (which makes them chemically changeable/dynamic, like bones) (Verna et al. 2003). Since no differences were shown, in certain groups a fusion of bone and tooth samples was used, yet the test group size was not increased, as only one bone or tooth sample was collected from each individual.
Breastfeeding
The effect of isotope fractionation during lactation raises the question of the extent to which oxygen isotope ratios for apatites in mothers and children with access to mother’s milk vary. As an extension of this subject, the present study verified if the mother’s intake of isotopically heavier water due to the application of specific culinary practices influenced the intensity of the process.
The analysis of isotope ratios in mothers and their breast-fed offspring in both models (tap water and boiled water) demonstrated that apatites in offspring were enriched with heavy oxygen isotopes in comparison to the values observed in their mothers. In the group of animals with access to tap water, δ18O values were 1.6‰ higher in breast-fed offspring than in females/mothers (median values), while for the group in which mothers drank boiled water the difference was 1.8‰. The different results from isotope fractionation, based on the assumption that mother’s milk had a higher oxygen isotope ratios in comparison to drinking water, led to an increase in the oxygen isotope value in infant tissues. This is a consequence of the fact that body water is enriched in heavier isotopes of oxygen in relation to drinking water, as lighter isotopes of 16O (Bryant and Froelich 1995 ) are excreted to a greater extent than heavier ones. Since milk is produced from water present in the body, it contains heavier isotopes than drinking water. Numerous studies have reported that isotope ratios of mother’s milk are higher than δ18O of water drank by mothers due to the presence of isotope fractionation in the physiological process of milk production. For example, in a study by Kornexl et al. (1997) the isotope ratio for cow milk was on average 2–6‰ higher than for drinking water made available to the animals. An experiment by Lin et al. (2003) showed that cow milk was on average 4% isotopically heavier than water, while in a study by Camin et al. (2008) a similar tendency corresponding to roughly 2‰ was observed. Until now, isotope analysis had not been performed for rodent milk. However, the findings of this study indicated that δ18Op in females/mothers and their offspring differed by approximately 1,6‰ and this difference was observed between maternal and offspring apatite. It is similar to differences observed in the literature between water and milk water (Kornexl et al. 1997).
An analysis of the isotope effect as a response to the change in the trophic level was reported both in the tap water model and the boiled water model, and its extent was similar (1.6‰ in the tap water model vs 1.8‰ in the boiled water model). Please note that for the former the difference is statistically significant, which is not the case with the latter. We may suspect that this is related to the fractionation factor (kinetic fractionation, which happens regardless of temperature) (Ustrzycka 2019) between mother’s milk and child’s body fluids. At birth, the isotope level for the offspring of a female who had drunk boiled water, due to the contact with mother’s bodily fluids throughout the gestation, was significantly higher (3.7‰ relative to the medians) than the isotope level for individuals from the same age group but born from a mother in the tap water model (not enriched to such extent in 18O). It seems that fluids/tissues enriched in a heavy isotope will be characterized by a lower fractionation level, being already saturated with a less reactive, heavier oxygen isotope.
Accordingly, in spite of the proved larger difference in isotope level between mothers and breastfed offspring in the boiled water group, the difference is not statistically significant, since the intervals in the upper part of dispersion overlap with the values reported for females drinking boiled water.
A detailed analysis performed separately for each female and her offspring from two groups established based on water type (Figures 3 and 4) demonstrates relatively large variability in terms of the quantitative difference in the isotope delta for individual females and breastfed rats. The maximum difference between a mother and her offspring was 2.5‰ in the tap water model and 3.2‰ in the boiled water model. Meanwhile, some young rats had less significantly elevated values, e.g., in the tap water model by a mere 1.2‰, albeit in the boiled water model there was a particular case in which the value dropped by 0.3‰ relative to the mother’s level. Studies on the teeth of various primates, including humans, also reveal a distinct fluctuation in the oxygen isotope ratio (∼1-2‰) in the offspring over the period of several weeks following birth, whereas heightened 18O values in tooth enamel in certain individuals were not observed altogether (Smith et al. 2022). Our study also reported cases in which the isotope ratio was lower in offspring than in their mother/female. This could be explained by the fact that several hours after birth the body of the newborn preferentially loses lighter oxygen isotopes (16O) as it starts to breathe on its own, transpire water through skin as well as lose liquids; moreover, body mass also decreases in the first days following birth (DiTomasso and Paiva 2018; Smith et al. 2022). Depending on the intensity of the aforementioned processes, for several weeks immediately after birth certain young individuals may compensate for the perinatal isotope loses.
Weaning process
Weaning is a process whereby mother’s milk is gradually replaced with food obtained from the environment (Dettwyler and Fishman 1992). The term “weaning age” signifies the moment in the life of a child when mother’s milk has been eliminated from the child’s diet (Williams et al. 2005). Stable isotopes of some elements (C, N, O) can detect the stages of the process. In the context of oxygen isotope analysis, we can consider weaning as a process of a gradual shift from the source of oxygen introduced into the body from the pool of oxygen present in mother’s milk to oxygen contained in weaning foods and drinks (containing environmental water). Accordingly, weaning age is the moment in which mother’s milk is entirely replaced by external water, which at the same time becomes the main source of oxygen incorporated in the individual’s bone tissue. In the case of the oxygen stable isotopes, it has the power of detecting the start of the weaning process because typical weaning foods such as paps, cereals, soups or juices and many others are prepared using external/ environmental/ drinking water.
In the group of animals with access to tap water and boiled water, we observed a drop in isotope ratios between breast-fed and completely weaned individuals (Figure 1). In this way, we observed the isotopic effect of oxygen related to the individual’s trophic level. The drop in the isotope composition after the start of weaning was also observed in oxygen isotopic studies of teeth from individuals from ancient populations in which isotopic proportions in teeth that are formed and mineralized asynchronously were compared (Wright and Schwarcz 1999; White et al. 2004a).
To date, there have been two publications that used analysis of oxygen isotope composition in bones to reconstruct weaning in ancient populations. In 2015, Britton et al. conducted research (White et al. 2004a; Britton et al. 2015) that outlined the need to verify whether analysis of stable oxygen isotopes in bone apatite may be used for the reconstruction of weaning age. Researchers analysed δ18Op in the bones of children and adults from a mediaeval archaeological site in Wharram Percy (England). In these publications, in children whose age was estimated at 3 years, the oxygen isotope ratio was higher by 1‰ on average compared to the bones of adult individuals and lower than the isotope delta for bone apatites in younger individuals. Based on archaeological material, these studies reported a drop in the oxygen isotope ratio in older children, which in the light of the present experiment confirms the hypothesis that this observation was an effect of weaning. The authors concluded that isotope analysis of bone tissue may be used to determine weaning age, considering that the interpretation of results needs to allow for the application of culture-specific routines pertaining to thermal processing. The second issue, i.e., the effect of thermal processing, was also analysed in this study.
The intensity of the effect of changes in the trophic level in the group of animals with access to tap and boiled water were analysed to determine the influence of isotope fractionation due to drinking water heating on the rate of change in isotope ratios of bone tissue in weaned individuals. With regard to the difference between the median isotope ratio in mothers and older weaned offspring (69 days after the start of weaning), we noticed that in the boiled water model, the difference (calculated on the basis of data from Table 2) was 1.1‰, which is lower than that of the tap water model (1.6‰). The results indicated that weaned individuals are close to attaining their mother’s isotope ratio, and hence, the level characteristic of the environmental water that they drank. This, however, does not mean that isotope proportions in tissues of rats that drank boiled water and were weaned, equalize more quickly with environmental water; an analysis of isotope ratios for breast-fed and weaned individuals showed that the difference in the tap water model was 0.5‰, while in the boiled water model it equaled 0.2‰ (Table 2). This means that the intake of thermally processed water after weaning did not affect oxygen isotope ratio in the offspring’s bone tissue – although in both models the isotope effect related to transition to extra-maternal oxygen source was noticeable.
A detailed analysis of individual “female vs offspring” groups indicates that the isotope ratio in the tissues of rats whose apatite was slightly enriched in heavy isotopes during breastfeeding lowered more rapidly and intensively after weaning. In other words, the greater the disproportion in δ18Op between mothers and their breastfed offspring, the less pronounced weaning-related isotope effect, which was observed independently of the type of water drunk by the female and her offspring after weaning.
An analysis of the possible application of the model to human populations
In isotope studies concerning humans, there is no exact knowledge of the discrepancy between the isotope composition of the apatites of the mother and breastfed offspring.
So far, anthropological and bioarcheological studies have not suggested what would be the sufficient extent of the differences in the isotope composition of oxygen in bone apatites of mothers and children to determine that it is either caused by breastfeeding or indicates another phenomenon, e.g., related to variability across individuals. To date, no isotope relationship between δ18O in mother’s milk, her body fluids, and the newborn’s tissue has been investigated. Little is yet known about the fractionation process of the physiological production of milk, the effect of hormones on isotope ratios in body fluids, and the tissues of individuals of various ages. Breastfeeding strategies and food preparation techniques in humans are so diverse that any generalisations of the phenomena of breastfeeding and weaning are impossible. The assumptions made in this study with regard to the model expected to translate the results of the experiment into information referring to humans was necessary due to the subject being insufficiently covered in research.
The literature offers tools, such as regression equations, describing the relationship between isotope composition of apatite phosphates and drinking water for various mammal species. Notably, values describing the isotope composition of bone phosphates are strongly correlated to the isotope ratio of drinking water, and although this relationship does not differ significantly, it is to a slight extent species-dependent; therefore, there are different regression equations for different animal species and humans (e.g., D’Angela and Longinelli 1990). Another key point in the context of the present study was the analysis of the relationship between the isotope composition of drinking and body water. It is commonly held that animal physiological water balance is reflected in the isotope signature of oxygen (Vander Zanden et al. 2016; Gregorčič et al. 2020). Oxygen isotope ratio in water contained in milk, as in environmental water, depends on temperature, humidity, and geographic coordinates of the location in which the animal lives, e.g., altitude above sea level (Abeni et al. 2015; Gregorčič et al. 2020).
The relationship between the isotope composition of oxygen in drinking water, body water, and bone phosphates relied upon in processing the results implies that human children fed only with the milk of their mother (who drinks thermally unprocessed water and eats thermally unprocessed food) may raise oxygen isotope proportions in their bones by approximately 2.7‰ (Figure 5). If the mother drinks only thermally processed liquids, the difference between the mother and her child, estimated for humans, may equal 0.4‰ (Figure 5). It appears that if the difference in oxygen delta between the mother and her offspring was smaller than in the tap water model, the fractionation had to “decelerate”, i.e., it must have been less intensive in locations where the environment was saturated with heavy isotopes. This suggests that differences between isotope values of mothers and breast-fed offspring may vary due to the intake of thermally processed liquids. Estimated for the human species, a reduced isotope effect in breastfed offspring (an increase by 0.4‰) related to the consumption of thermally processed, heavy isotope food by the mother (which translates into milk with heavier isotopes) differs from the results obtained for rats (an increase by 1.8‰) and requires additional research.
Scientific research show that human and rat milk have a similar qualitative composition. Comparative chromatography of human, cow, rat, and pig milk proved that human and rat milk displayed the smallest differences (Roberts et al. 1954). In the same study, it was reported that apart from lactose, which was obviously present in the milk of all species concerned, glucose was detected only in humans and rats. It appears, however, that the discrepancies observed in the experiment and in the estimation for humans (tap water vs boiled water model) arise from different relationships between the isotope values for drinking and body water, including rat milk and human milk, but also from various other factors, such as physical activity (e.g., Bryant and Froelich 1985). The authors would like to stress that any attempts to use animal models for interpretation in bioarchaeological studies are always highly challenging and tainted by uncertainty. Therefore, the results of the analysis concerning the application of the results of the experiment to human population should be approached with considerable caution.
It is rather difficult to discuss the results in reference to literature because it is the first research of this type on fragments of fresh skeletons. Variability may only be evaluated by contrasting the resulting data with data available in literature on BWP studies carried out on other- archaeological skeletal material (Table 3).
The rise in oxygen isotope ratios in tissues of offspring in relation to adult individuals has been studied in many archaeological studies (e.g., Williams et al. 2005; Britton et al. 2015; Smith et al. 2022). In studies on archaeological material, disproportions in the isotope composition of oxygen between bone apatite in children and women or adult individuals varied. For example, in a study on the skeletons of adults and children under the age of 3 years, White et al. (2004a) reported a 2‰ difference in isotope delta. Research by Wright and Schwarcz (1998) involved a comparison of the value of isotope delta in the tissue of first molars that mineralize from birth to the age of 3, premolars that mineralize between the age of 2 and 6, and M3s that start to mineralize around age 9 (Wright and Schwarcz 1998). However, in this case, findings reported by researchers show that the difference between δ18O for tooth phosphates at the age when children were breast-fed and the period when their diet was likely similar to the one typical of adults was only 0.6 to 0.7‰. Other studies performed on archaeological dental material revealed that the oxygen isotope delta for hydroxyapatite carbonate groups differed by 0.5‰ across deciduous and permanent dentition (Dupras and Tocheri 2007). Perhaps this is where we may observe the effect of boiling liquid food and beverages on the variation of isotope values between adult and child tissues. It seems that a reliable method of studying child diets is the method using incremental dentine collagen samples, which to a substantial extent allows us to avoid uncertainties related to changes in nitrogen isotope values due to, e.g., perinatal stress or the mother’s non-standard diet. However, even this method is not perfect. Research on deciduous teeth in contemporary populations of children from Bradford (UK) proved that the dentine of children fed with modified milk until 9 months of age did not reveal any nitrogen isotope variability, whereas breastfed children had a wider range of nitrogen isotope values, reflecting their mothers’ diverse diet. In addition, some children breastfed until 6 months of age still showed increased nitrogen isotope values after the introduction of supplementary food (Beaumont 2020).
Table 5 contains data from available studies that analysed the weaning and breastfeeding process in human and nonhuman individuals with the use of oxygen isotopes. In archaeological studies of human populations, the difference between oxygen isotope ratios for potentially breastfed offspring and adult individuals is a rather extensive range from 0.5‰ to 2‰ (Table 5). This range includes also other species of primates (Smith et al. 2022), and even the results of analysis for the extinct woolly mammoth (Metcalfe et al. 2010). The maximum difference between oxygen isotope ratio in literature is 2‰ but we are aware that in uncontrolled studies we should include various factors which may affect interpretation.
| Increase of δ18O child vs mother | Material | Species | Authors, year |
| ∼0.7‰ | enamel carbonates | humans | Roberts et al. 1988 |
| ∼0.7‰ | enamel carbonates | humans | Wright & Schwarcz 1999 |
| ∼1.2‰ | enamel phosphates | humans | White et al. 2004a |
| ∼2‰ | carbonates of molar teeth enamel | woolly mammoth | Metcalfe et al. 2010 |
| ∼0.5-0.7‰ | bone carbonates | humans | Williams et al. 2005 |
| ∼1.2‰ | bone phosphates | humans | Britton et al. 2015 |
| ∼1-2‰ | prenatal vs postnatal enamel of M1 | humans, other primates | Smith et al. 2022 |
| ∼1.6‰ | bone and teeth phosphates | rats | this research, tap water model |
| ∼2.7‰ | bone phosphates | humans | estimation-this research, tap water model |
We must emphasize that increased isotope values in offspring that we may suspect as having been weaned does not necessarily mean that they continued to drink mother’s milk. Higher isotope ratios may result from feeding thermally processed food to children, especially if we consider that paps, gruels, or soups were prepared by boiling cereal flakes and grains in water. Indian sources from 6th century BC to 1st century AD suggest that weaning started by feeding the child with boiled rice (Fildes 1986). In the 15th century, doctors recommended that children weaned before the end of the first year of life should be fed with boiled goat milk with water and a pap made of boiled water, bread, and honey (Fildes 1986). In analysis of the prevalence of scurvy in medieval and early modern populations, Krenz-Niedbała (2016) concluded that weaned children and their parents ate mostly meals based on cereals and boiled vegetables. The author observed that scurvy was not such a severe problem in the area of today’s Poland as suggested in historical sources, and explains that this was thanks to a technique used by historical populations that involved boiling meals slowly and for an extended period of time. This method enabled them to preserve, in an unchanged form, at least 50% of the vitamins contained in vegetables. Water used in making meals for weaned children could additionally be isotopically enriched not only by boiling, but also by being stored by human groups inhabiting dry areas (Perry et al. 2020).
Thus, many factors may modify the breastfeeding effect by enriching or depleting offspring’s tissues in the heavier isotope 18O. However, as demonstrated in the experiment, oxygen isotope value in the apatites of the offspring fed by milk from female rats who had drunk thermally processed water was as much as 3.7‰ higher than in rats fed by females from the tap water group. This finding proves that the intake of liquids which have been boiled or otherwise culturally enriched in heavy oxygen isotopes has a substantial impact on the isotope ratios in the bones of both mothers and their breastfed offspring and that it is highly probable that this holds true not only for bones but may be of significance for isotope studies analysing teeth.
Conclusions
The high complexity of the breastfeeding and weaning processes prompts researchers to ask many questions. How do cultural practices involving thermal processing of food and drink influence the interpretation of results? Are human groups which less often prepared food with the use of fire characterized by a greater difference between δ18O in mothers and breastfed children than populations whose diet is strictly based on boiling and other thermal processing methods? Could this be correlated with the environmental conditions in which a given community functioned (latitude, climate)? Does the inaccessibility of odontological material combined with diagenesis of collagen preclude the reconstruction of infant feeding strategies? On the basis of this study, it may be argued that, despite multiple unfavourable issues, in exceptional cases oxygen isotopes analysed in bone phosphates can be used in BWP studies and effectively supplement other methods. Caused by breastfeeding and weaning, the change in the oxygen isotope level in bone phosphates is noticeable, and thermal processing is a factor which bolsters the effect. It was estimated that breastfeeding can raise oxygen isotope level in a human child’s bones by 2.7‰ relative to its mother’s level. If the mother consumes only thermally processed food, the difference may be smaller, approximating 0.4‰.
In the light of many assumptions which have been made in this study, our work does not constitute a ready-made solution to be applied in research on children’s diet, yet, undoubtedly its experimental section emphasises the need to find more information on the basis of the BWP phenomenon so that isotope analyses (including oxygen isotope analyses) could be employed as broadly as possible and provide research opportunities in the absence of odontological material, with results that could be reliably interpreted.
Acknowledgements
This research was supported by the National Science Centre (Poland) [grant number 2014/15/N/NZ8/00351]. We would like to thank the staff from The Department of Neuroanatomy Jagiellonian University, especially Zuzanna Setkowicz and Małgorzata Kaczyńska for donating the animal tissues used in this study.
Conflict of interests
All Authors declare no conflict of interests.
Authors’ contribution
AL-G: conceived and designed the analysis, collected the data, contributed data or analysis tools, performed the analysis, wrote the paper, review and editing the paper; BC-S: involved in planning the work, review and editing the paper; MF: review and editing the paper; KS: involved in planning and supervised the project, aided in interpreting the results, review and editing the paper.
* Corresponding author: Beata Cienkosz-Stepańczak, Institute of Zoology and Biomedical Research, ul. Gronostajowa 9, 30-387 Krakow, Poland, e-mail: b.stepanczak@uj.edu.pl
 https://orcid.org/0000-0002-3613-353X
https://orcid.org/0000-0002-3613-353X

