
Available online at: https://doi.org/10.18778/1898-6773.85.4.09
Division of Anatomy, Wroclaw Medical University, Wroclaw, Poland
Faculty of Health Sciences, Wroclaw Medical University, Wroclaw, Poland
Clinical and Dissecting Anatomy Students Scientific Club, Faculty of Medicine, Wroclaw Medical University, Wroclaw, Poland
Division of Anatomy, Wroclaw Medical University, Wroclaw, Poland
Faculty of Stomatology, Wroclaw Medical University, Wroclaw, Poland
Division of Anatomy, Wroclaw Medical University, Wroclaw, Poland
ABSTRACT: Introduction: The Flexor Carpi Ulnaris (FCU) is a part of the palmar the forearm muscle group and one of the most important muscles for upper limb functioning - is responsible for flexion and adduction of the hand at the radio-carpal joint. There are clinically significant but rare anatomical variations of FCU. The variability of the FCU has not been described up to now, and no typology of the muscle based on its more variable terminal attachment has been created.
Aim of the study: Determination of FCU muscle typology based on available fetal material.
Material and methods: A total of 114 human fetuses (53 female, 61 male) between 117 and 197 days of fetal life were eligible for the study. Preparations were carried out using classical anatomical techniques based on a previously published procedure. Thanks to that significant anthropometric landmarks were visible for the gathering of metric measurements. Metric measurements were taken and statistically analysed using R-Project software.
Results: A new typology was created based on variable muscle insertions. Additionally, the presence of an atypically located, additional, separated muscle belly was described. A comparison of measurements of the left upper limb in relation to the right upper limb showed significant differences for forearm length to the anthropometric point of the stylion radiale, limb length, total FCU length and FCU length which means that the left limb is longer than the right limb. A comparison of FCU insertion types between left and right upper limb showed there’s no significant difference between counts of each type.
Conclusion: The FCU is a muscle that is easy to palpate and may therefore act as a topographical marker for healthcare professionals. Knowledge of its variability is not only of theoretical importance but also has clinical significance. The current publication demonstrates presence of variability in FCU terminal attachment. Certainly, this topic requires further research and continued work on a detailed understanding of forearm anatomy in the fetal period.
KEY WORDS: fetal anatomy, forearm muscles, dissection, cadavers, flexor carpi ulnaris
The Flexor Carpi Ulnaris (FCU) is one of the important muscles of the forearm (Kreulen et al. 2004). Anatomically, it is a part of the palmar forearm muscle group. It is located medially in the superficial layer of the forearm muscles (Budoff et al. 2005). The proximal attachment of the muscle is based on two heads – the humeral head, which begins on the medial epicondyle of the humerus, and the ulnar head, the origin of which is located on the posterior wall of the olecranon. It is worth noting that the initial part of the muscle is also closely connected with the surrounding fascia of the forearm. (Ziajkiewicz et al. 2010). The muscle passes downward, parallel to the flexor digitorum superficialis. Then it turns into a long tendon attached to the pisiform and proximal part of hamate bone (Fridén et al. 2004).
Physiologically, that muscle is responsible for flexion and adduction of the hand at the radio-carpal joint. In addition, the humeral head supports the flexion at the elbow joint (Esplugas et al. 2016). The evolution of hominids ultimately led to the adoption by humans of an upright position. This lead to changed characteristics of the fore limb. It lost its locomotor function and became an upper limb – a manipulative tool (Capdarest-Arest et al. 2014; Marzke 1997; Skinner et al. 2015). The change in the function of this organ required some adjustment in the musculature of the limb. This became especially evident in the distal part of the limb – data clearly indicate the different dynamics of muscle development in the proximal and distal parts of the upper limb. The muscles of the forearm develop later in the embryonic period. This may contribute to greater variability in their terminal attachments as well as increased variation in the structure of the entire muscle (Bobzin et al. 2021; Giuliani Piccari Scarpa et al. 1977; Guéro 2018).
These assumptions are somewhat confirmed by observations from a number of “case-studies” that indicate the presence of a number of rare anatomical variations of FCU. Some of these are clinically significant. For example, a 1992 study showed a case of FCU terminal tendon duplication leading to ulnar nerve dissection (al-Qattan, Duerksen 1992). In contrast, authors from the Czech Republic, for example, demonstrated the significant importance of additional detached FCU fragments as a cause of increased risk of iatrogenic complications (Kunc et al. 2019). Similar observations are also shown by authors from Australia (Ang et al. 2010).
On the other hand, on a large material of matures/senilis specimens, the authors showed a relatively high stability of the FCU course (Loth 1931). The stability of the course and of proximal and distal attachments is emphasized by many authors of papers from the late 19th and early 20th centuries (Loth 1912; Wood 1867).
The historical scientific data are somewhat at odds with the new literature reports indicating the presence of quite a lot of variability in FCU.
This noticeable difference in the frequency of FCU variation is perhaps the result of the microevolution process described in the available literature (Kralik, et al. 2017; Pelletier and Coltman 2018). The result of the accumulation of single mutations as a cause of the observed variability cannot be excluded either. Indeed, the available scientific data indicate that the neonatal genome currently contains, on average, more than 70 new mutations (Conrad et al. 2011).
Therefore, in the opinion of the authors of this paper, an analysis based on a larger number of cases is necessary. Recent literature reports are mainly on the evaluation of single case – studies (Ata et al. 2018; Bhardwaj et al. 2013; Pressney et al. 2020; Yamamoto et al. 2021).
Based on the research data that were presented, an attempt was made to assess the anatomical variability of FCU. Due to very limited access to cadavers of adult individuals, it was decided to carry out such an analysis based on fetal material. In the past, a scientific team from our Unit has already carried out a metric assessment and evaluation of the growth dynamics of FCU (Ziajkiewicz et al. 2010).
However, the variability of the FCU has not been determined up to now, and no typology of the muscle has been created based on its more variable terminal attachment.
It is worth noting that an additional but scientifically significant reason for the choice of such material is also the fact of a much later formation of the FCU in the fetal period, which may translate into the presence of greater variability in the structure of the muscle.
Determination of FCU muscle variations based on available fetal material.
A total of 114 human fetuses (53 female, 61 male) between 117.0 and 197.0 (median 177.0) days of fetal life were eligible for the study. The material came from the fetal collection stored in the fetal laboratory of the Division of Anatomy.
Basic metrics characterizing the study sample of fetuses are included in Table 1.
Table 1. Characteristics of the study sample of featuses
| feature | N | min | max | SD |
| age.morph.day | 73 | 117.0 | 197.0 | 177.93 |
| age.cal.day | 67 | 68.0 | 254.0 | 166.63 |
| arm.leng.R | 114 | 32.9 | 68.2 | 54.97 |
| arm.leng.L | 114 | 32.9 | 68.3 | 54.82 |
| for.leng.R | 114 | 26.2 | 54.2 | 43.87 |
| for.leng.L | 114 | 26.2 | 57.8 | 44.27 |
Abbreviaions: age.morph.day – featus morphological age in days; age.cal.day – featus calendar age in days; arm.leng.R – length of right arm, arm.leng.L – length of left arm; for.leng.R – length of right forearm; for.leng.L – length of left forearm; N – total number of individuals; min – minimal value in centimeters; max – maximal value in centimeters; SD – standard deviation
Fetal material was obtained from local gynecological clinics between 1960 and 1996. The fetuses were from unplanned preterm births or miscarriages. The course of delivery as well as the decision to stop resuscitating the fetus was made by a medical team independent of the researchers.
The fetuses were stored in a dedicated preservative fluid containing formaldehyde, ethyl alcohol and glycerol in fixed proportions. The material was stored in a darkened room – a specialized laboratory with constant temperature and no exposure to light. The method of storing and preserving the material did not change throughout the period from the acquisition of the material to its use for scientific purposes (Karykowska et al. 2021; Suchanecka et al. 2022; Ziółkowski et al. 1994).
Fetuses with apparent anatomical and developmental abnormalities and those without basic clinical and morphological documentation were excluded from the study. Material with secondary damage and deformities due to improper storage was also excluded. The scientific value and reliability of the fetal collection has been confirmed in many previous scientific publications (Dudek et al. 2018; Gworys and Domagała 2003; Karykowska et al. 2022a; Karykowska et al. 2022b; Kędzia et al. 2022; Wozniak et al. 2019).
The study was conducted between October 2020 and March 2022. Preparations were carried out using classical anatomical techniques based on a previously published scheme of procedure (Suchanecka et al. 2022). It was based on removing the skin and subcutaneous tissue, then gaining full access to the fascia of the forearm to cut it and open the anterior compartment.
The next aim of the dissection was to make significant anthropometric points visible for the gathering of metric measurements.
A detailed dissection of the Flexor Carpi Ulnaris muscle was then carried out (shown on Figure 1), so that the planned metric measurements could be carried out in a reproducible and reliable manner. In the final stage of the study, the location of the initial attachment as well as the end attachment of the muscle was assessed in order to classify the individual case into the typology that had been created earlier.

Fig. 1. Dissection stages of Flexor Carpi Ulnaris on fetal cadavers
The phases of the scientific work were documented with schematic figures and standard photographs. Photographs were taken with a digital system from Tagarno Prestige (Tagarno Innovision A/S, Denmark) and with a Sony Alfa 7II digital camera (Minato, Tokyo, Japan) stabilised on a Manfrotto tripod (Vitec Group, Richmond,UK). Metric measurements were taken using a Mitutoyo Absolute Digimatic digital calliper (Mitutoyo Corporation, Kanagawa, Japan). Each metric measurement was taken three times by two independent observers (KS, MS) and an average was then calculated from the results, which formed the basis for further analysis. Statistical analysis was performed using R-Project software (The R Foundation for Statistical Computing, Vienna, Austria).
Data of each fetus were obtained from two sources:
A significance level of α = 0.05 was assumed. All collected numerical values were summarized using: mean, median, min, max and 95% CI for mean. A Shapiro-Wilk test was performed for each variable in order to check if it is normally distributed. The same calculations were performed after dividing the data into two groups: females and males. A comparison between sexes was done with a Mann-Whitney test (because most of the variables were not distributed normally). Measurements of the left upper limb were compared against measurements of the right upper limb using Wilcoxon signed rank test. Linear regression coeffcients between respective variables of left and right side were also calculated. We did not assume a priori any correlations in each pair of 31 numeric variables: our aim was to find such. For each unique pair of collected variables a scatter plot with a linear regression line was generated. The number of created plots was large: The entire study protocol was approved by the local bioethics committee (KB167/21).
Based on the conducted analysis, a new typology was created, due to muscle insertion.
Type1 – insertion to pisiform bone
Type 2 – insertion to 4th metacarpal bone
2a – inserted medially
2b – inserted laterally
Type 3 – insertion to 5th metacarpal bone
3a – inserted medially
3b – inserted laterally
Type 4 – insertion to flexor retinaculum
Exact appearance of every mentioned type is shown on Figure 2.
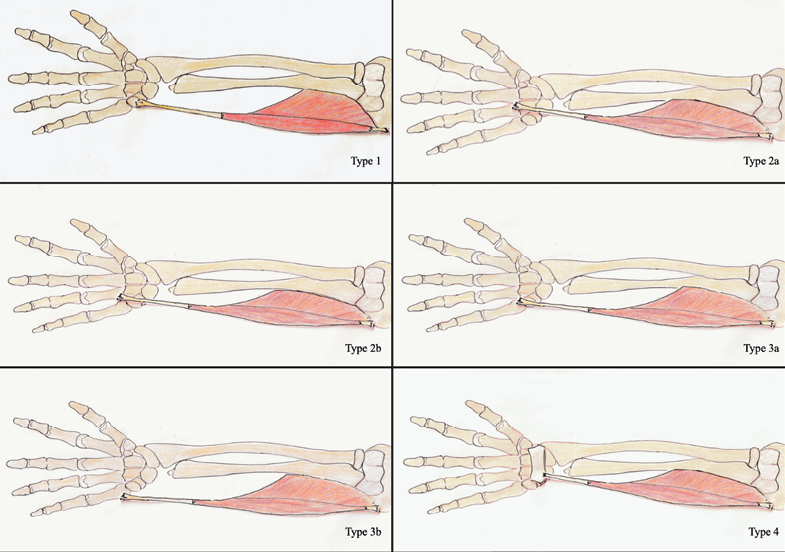
Fig. 2. Exact appearance of each mentioned Flexor Carpi Ulnaris type
Additionally, in individual cases, the presence of an atypically located, additional, separated muscle belly located under the main FCU mass, running from the area of the medial epicondyle of the humerus to the pisiform bone was described.
It has been shown that in the most cases type 1 is dominant. Other types occur with a statistically lower frequency than type 1 – shown on Table 2.
Table 2. Frequency of different types of FCU due to its distal attachment
| Type variation | Left | Right | ||
| Frequency | % | Frequency | % | |
| Type 1 | 78 | 68.42 | 81 | 71.05 |
| Type 2a | 6 | 5.26 | 7 | 6.14 |
| Type 2b | 9 | 7.89 | 8 | 7.02 |
| Type 3a | 11 | 9.65 | 7 | 6.14 |
| Type 3b | 7 | 6.14 | 9 | 7.89 |
| Type 4 | 3 | 2.63 | 2 | 1.75 |
For the most of numerical variables Shapiro-Wilk tests rejected the null hypothesis that a variable is normally distributed. However, interestingly, this does not apply to female fetuses in which for about a half number of variables the normality test failed to reject the null hypothesis. We do not know any plausible explanation. Further statistical tests rejected the hypothesis that there is a difference between females and males for each of 31 numerical variables.
Comparisons of the left upper limb measurements versus the right upper limb showed significant differences for: forearm length to stylion radiale anthropometric point, the length of the hand the total length of FCU and length of FCU belly. For each measurement pair of the analogous quantities in left and right upper limb a scatter plot with an added linear regression line was created. Almost all plots (12 out of 13) showed a significant, strong (r > 0.85) positive linear relationship; in 10 out of 13 plots the coefficient r was greater or equal to 0.90. Only the relationship between FCU tendon length (left vs right) was moderate (r = 0.50), which can be easily explained by an overly vague definition of how this measurement should be performed. Our attempt to find meaningful relationships between other quantities was successful. From 465 analysed pairs of variables, we found 44 pairs which showed a statistically significant strong positive linear relationship. What is interesting and unexpected for us is that among these 44 relationships, there is only one in which the fetus’ mass is present and only one which considers the fetus’ age. For categorical variables the chi-square tests rejected, with an ifinitesimaly low p-values, that types of FCU insertions are equally distributed, both in left and right upper limb.
A comparison of FCU insertion types between left and right upper limb showed there is no significant difference between counts of each type. This statement holds also for males and females considered separately.
The ulnar specific pathway formed by the FCU but also the flexor digitorum profundus as well as the flexor digitorum superficialis is ‘used’ by the ulnar vessels and nerves as a pathway from the humero-ulnar joint area towards the hand (Grechenig et al. 2000).
The FCU is a muscle that is easy to palpate and may therefore act as a topographical marker for healthcare professionals in the search for local neurovascular bundle. Some researchers suggest that the FCU is an important anatomical landmark for surgeons and ultrasonographers. Knowledge of the variability of the terminal segment of this muscle is therefore not only of cognitive importance but also of profound clinical significance (Mizia et al. 2021).
Due to the limited access to anatomical specimens of adults, there was a strong need to look for alternative sources of biological material (Ghosh 2017; McCumber et al. 2021). Fetal collections assembled in many anatomical laboratories in the 19th and 20th centuries could be such a source (Rohan et al. 2019).
In Poland, due to the high fertility rate and, at the same time, the underfunding of health care during the communist period between 1960 and 1990, the Department of Anatomy was enriched with a collection of genetically defect-free fetuses. This material is unique. The socio-economic progress and advances in medical science have resulted in low mortality rate of fetuses and newborns in Poland today (Krzyżak et al. 2014; Troszyński et al. 2009; Wiśniewski et al. 2019).
The fetal material, due to its much smaller size, is easier to store. Thus, it is possible to collect a large number of foetuses, which increases the chances of conducting a reliable statistical analysis of the results and conducting research on a relatively large study material. In the case of the study described here, anatomical analysis was carried out using 114 fetuses from the local anatomical collection.
It showed a stable course of FCU, which is consistent with the standard textbook description. The initial attachment in all cases is typical and the terminal tendon attaches in a standard manner in most cases.
Similar observations are shown by Loth and Wood evaluating FCU on adult material (Loth 1931; Wood 1866).
It should be noted, that the current publication demonstrates the presence of variability in FCU terminal attachment. Various types of the distal attachment and their percentages are shown on Figure 3.
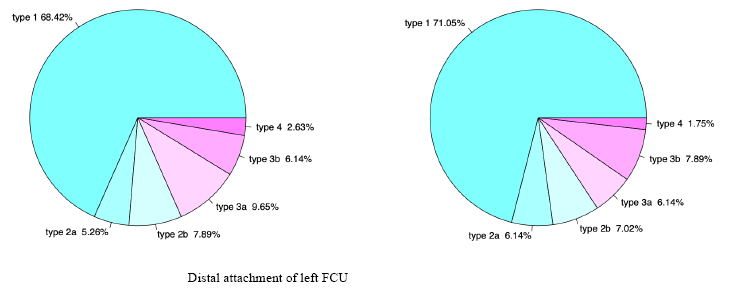
Fig. 3. Various type of the distal attachment of Flexor Carpi Ulnaris
An interesting novelty compared to previous studies was the demonstration of the presence of a statistically significant difference in selected bilateral measurements with a statistically significant difference in favor of the left upper limb. Due to the established measurement procedure – two independent researchers take the same measurements three times and the average of all these measurements is included in the analysis – the authors are of the opinion that the differences shown are not a result of measurement errors. It is worth noting that the individual researchers did not have access to data collected by the second author.
Similar differences were shown in the work concerning the flexor carpi radialis (Suchanecka et al. 2022).
A relationship between the variation shown and intrauterine limb developmental limitations or atypical response to the preservative material used or specific fetal developmental disorders cannot be excluded either. There are hypotheses in the literature that suggest that the lateralization process of the brain is initiated already in fetal life, which may indirectly explain the demonstrated differences.
However, other factors related to human evolution must be taken into account. For example, Saniotis et al. (2021) point out that the directional factors of evolution are mutations and natural selection. According to the authors of this publication, it cannot be excluded that the observed variation in selected muscles is the result of a single mutation that disrupts or alters the process of fetal development.
It has been proved in the case of muscles that a reprogramming of gene transcription is associated with remodeling of the contractile properties of the fibers (slow, fast fibers) and a remodeling of the metabolic profile of the muscle (Aleman et al. 2022; Baumert et al. 2018).
Whether single mutations can contribute to increased variability in muscle attachment - this question is difficult to answer unequivocally. Certainly, genetic disorders affect skeletal muscle function in animals in such a way that atypical-looking muscle fibers are formed (Yuan et al. 2021). Thus, it is possible to hypothesize a potential link between mutations and muscle variability (Krämer et al. 2006).
You et al. (2022) conclude that mutations are an inevitable consequence of the poor quality of the chemical bonds between the bases that make up DNA. Their number is gradually increasing in humans due to the species’ increased exposure to mutagenic agents as well as better health protection.
Improving intrauterine as well as extrauterine health care reduces the opportunity for natural selection to operate. As a result of reduced evolutionary pressure, the number of mutations and developmental disorders are increasing - and this may also be translated into the observed higher variability and altered symmetry when compared to the past.
Certainly, this topic requires further research and continued work on a detailed understanding of forearm anatomy in the fetal period.
Limitations:
Acknowledgements
The authors would like to thank Ms Alina Proniewicz for her assistance with the project. The study was supported by statutory grant SUBZ.A351.22.038 (The presented research results, carried out within the framework of the topic according to the register in the S system with the number SUBZ.A351.22.038, were financed from the subsidy granted by the Minister of Science and Higher Education).
In accordance with the guidelines of the editors of anatomical journals (Iwanaga et al. 2022).
The authors sincerely thank those who donated their bodies to science so that anatomical research could be performed. Results from such research can potentially increase humankind’s overall knowledge that can improve patient care. Therefore, these donors and their families deserve our highest gratitude.
Conflict of interests
The authors declared no conflict of interest.
Authors’ contributions
KS was project supervisor, conceived the paper and co-edited the final version of the manuscript; RK was responsible for the preparation of the foetal material and literature selection; AM was responsible for the preparation of the foetal material and writing the final version of the manuscript; JC collected the data and performed statistical computations; JU assisted with writing the manuscript and was responsible for figures creating; MS was responsible for the preparation of the fetal material and literature selection; all authors substantially contributed to revisions.
*Corresponding author: Małgorzata Suchanecka, Division of Anatomy, Wroclaw Medical University, Wroclaw Poland, Phone: 717841331, fax: 717840079; e-mail: malgorzata.suchanecka@umw.edu.pl
al-Qattan MM, Duerksen F. 1992. A variant of flexor carpi ulnaris causing ulnar nerve compression. J Anat 180 (Pt 1):189–90.
Aleman M, Scalco R, Malvick J, Grahn RA, True A, Bellone RR. 2022. Prevalence of Genetic Mutations in Horses With Muscle Disease From a Neuromuscular Disease Laboratory. J Equine Vet Sci 118:104129.
Ang GG, Rozen WM, Vally F, Eizenberg N, Grinsell D. 2010. Anomalies of the flexor carpi ulnaris: clinical case report and cadaveric study. Clin Anat 23(4):427–30.
Ata AM, Kara M, Aydin G, Kaymak B, Gürçay E, Özçakar L. 2018. Ultrasound Imaging for Muscle Variations: Digastric Flexor Carpi Ulnaris, Gastrocnemius Tertius, and Supernumerary Fibularis Longus in an Asymptomatic Family. Am J Phys Med Rehabil 97(11):e107–09.
Baumert P, G-REX Consortium, Stewart CE, Lake MJ, Drust B, Erskine RM. Variations of collagen-encoding genes are associated with exercise-induced muscle damage. 2018. Physiol Genomics. Sep 1;50(9):691–693.
Bhardwaj P, Bhandari L, Sabapathy SR. 2013. Supernumerary flexor carpi ulnaris–case report and review. Hand Surg 18(3):393–97.
Bobzin L, Roberts RR, Chen HJ, Crump JG, Merrill AE. 2021. Development and maintenance of tendons and ligaments. Development 148(8):dev186916.
Budoff JE, Kraushaar BS, Ayala G. 2005. Flexor carpi ulnaris tendinopathy. J Hand Surg Am 30(1):125–29.
Capdarest-Arest N, Gonzalez JP, Türker T. 2014. Hypotheses for ongoing evolution of muscles of the upper extremity. Med Hypotheses 82(4):452–56.
Conrad DF, Keebler JE, DePristo MA, Lindsay SJ, Zhang Y, Casals F, et al. 2011. 1000 Genomes Project. Variation in genome-wide mutation rates within and between human families. Nat Genet Jun 12;43(7):712–4.
Dudek K, Nowakowska-Kotas M, Kędzia A. 2018. Mathematical models of human cerebellar development in the fetal period. J Anat 232(4):596–603.
Esplugas M, Garcia-Elias M, Lluch A, Llusá Pérez M. 2016. Role of muscles in the stabilization of ligament-deficient wrists. J Hand Ther 9(2):166–174.
Fridén J, Lovering RM, Lieber RL. 2004. Fiber length variability within the flexor carpi ulnaris and flexor carpi radialis muscles: implications for surgical tendon transfer. J Hand Surg Am 29(5):909–14.
Ghosh SK. 2017. Paying respect to human cadavers: We owe this to the first teacher in anatomy. Ann Anat 211:129–34.
Giuliani Piccari Scarpa G, Marchini M, Nicoletti P. 1977. Osservazioni sullo sviluppo dell’articolazione scapolo-omerale nell’uomo, con particolare riferimento ai suoi rapporti con il tendine del capo lungo del muscolo bicipite del braccio. Arch Ital Anat Embriol 82(1):85–98.
Grechenig W, Clement H, Egner S, Tesch NP, Weiglein A, Peicha G. 2000. Musculo-tendinous junction of the flexor carpi ulnaris muscle. An anatomical study. Surg Radiol Anat 22(5–6):255–60.
Guéro S. Developmental biology of the upper limb. 2018. Hand Surg Rehabil 37(5):265–74.
Gworys B, Domagala Z. 2003. The typology of the human fetal lanugo on the thorax. Ann Anat 185(4):383–86.
Iwanaga J, Singh V, Takeda S, Ogeng’o J, Kim HJ, Moryś J et al. 2022. Standardized statement for the ethical use of human cadaveric tissues in anatomy research papers: Recommendations from Anatomical Journal Editors-in-Chief. Clin Anat 35(4):526–528.
Karykowska A, Domagała ZA, Gworys B. 2022. Musculus peroneus longus in foetal period. Folia Morphol (Warsz) 81(1):124–33.
Karykowska A, Domagala ZA, Gworys B. 2022. Topography of the common fibular nerve terminal division in human foetuses. Folia Morphol (Warsz) 81(1):37–43.
Karykowska A, Rohan-Fugiel A, Mączka G, Grzelak J, Gworys B, Tarkowski V, Domagała Z. 2021. Topography of muscular branches of the superficial fibular nerve based on anatomical preparation of human foetuses. Ann Anat 237:151728.
Kędzia A, Dudek K, Ziajkiewicz M, Wolanczyk M, Seredyn A, Derkowski W, Domagala ZA. 2022. The morphometrical and topographical evaluation of the superior gluteal nerve in the prenatal period. PLoS One 17(8):e0273397.
Králík M, Ingrová P, Kozieł S, Hupková A, Klíma O. 2017. Overall trends vs. individual trajectories in the second-to-fourth digit (2D:4D) and metacarpal (2M:4M) ratios during puberty and adolescence. Am J Phys Anthropol Apr;162(4):641–656.
Krämer DK, Ahlsén M, Norrbom J, Jansson E, Hjeltnes N, Gustafsson T, et al. 2006. Human skeletal muscle fibre type variations correlate with PPAR alpha, PPAR delta and PGC-1 alpha mRNA. Acta Physiol (Oxf) 188(3-4):207–16.
Kreulen M, Smeulders MJ, Hage JJ. 2004. Restored flexor carpi ulnaris function after mere tenotomy explains the recurrence of spastic wrist deformity. Clin Biomech (Bristol, Avon) 19(4):429–32.
Krzyżak M, Maślach D, Piotrowska K, Charkiweicz AE, Szpak A, Karczewski J. 2014. Perinatal mortality in urban and rural areas in Poland in 2002-2012. Przegl Epidemiol 68(4):675–79.
Kunc V, Stulpa M, Feigl G, Kachlik D. 2019. Accessory flexor carpi ulnaris muscle with associated anterior interosseous artery variation: case report with the definition of a new type and review of concomitant variants. Surg Radiol Anat 41(11):1315–18.
Loth E. 1912. Beiträge zur Anthropologie der Negerweichteile (Muskelsystem). Strecker & Schröder.
Marzke MW. 1997. Precision grips, hand morphology, and tools. Am J Phys Anthropol 102(1):91–110.
McCumber TL, Latacha KS, Lomneth CS. 2021. The state of anatomical donation programs amidst the SARS-CoV-2 (Covid-19) pandemic. Clin Anat 34(6):961–65.
Mizia E, Pekala PA, Skinningsrud B, Rutowicz B, Piekos P, Baginski A, Tomaszewski KA. 2021. The anatomical landmarks effective in the localisation of the median nerve during orthopaedic procedures. Folia Morphol (Warsz) 80(2):248–54.
Pelletier F, Coltman DW. 2018. Will human influences on evolutionary dynamics in the wild pervade the Anthropocene? BMC Biol. Jan 15;16(1):7.
Pressney I, Upadhyay B, Dewlett S, Khoo M, Fotiadou A, Saifuddin A. 2020. Accessory flexor carpi ulnaris: case report and review of the literature. BJR Case Rep 6(3):20200010.
Rohan A, Domagała Z, Abu Faraj S, Korykowska A, Klekowski J, Pospiech N, Wozniak S, Gworys B. 2019. Branching patterns of the foetal popliteal artery. Folia Morphol (Warsz) 78(1):71–78.
Saniotis A, Henneberg M, Mohammadi K. 2021. Genetic load and biological changes to extant humans. J Biosoc Sci Jul;53(4):639–642.
Skinner MM, Stephens NB, Tsegai ZJ, Foote AC, Nguyen NH, Gross T, Pahr DH, Hublin JJ, Kivell TL. 2015. Human evolution. Human-like hand use in Australopithecus africanus. Science 347(6220):395–99.
Suchanecka M, Siwek K, Ciach J, Eicke K, Tarkowski V. 2022. Typology of flexor carpi radialis muscle in human fetuses. Folia Med Cracov 62(1):5–17.
Troszyński M, Niemiec T, Wilczyńska A. 2009. Ocena funkcjonowania trójstopniowej selektywnej opieki perinatalnej na podstawie analizy umieralności okołoporodowej wczesnej i cieć cesarskich w Polsce w 2008 roku [Assessment of three-level selective perinatal care based on the analysis of early perinatal death rates and cesarean sections in Poland in 2008]. Ginekol Pol 80(9):670–677.
Wingate Todd T. 1931. Anthropologie des parties molles. E LOTH.. Pages vii + 539. Masson et Cie. The Anatomical Record 51(2):219–22.
Wiśniewski M, Baumgart M, Grzonkowska M, Szpinda M, Pawlak-Osińska K. 2019. Quantitative anatomy of the ulna’s shaft primary ossification center in the human fetus. Surg Radiol Anat 41(4):431–39.
Wood J. 1866. Variations in Human Myology Observed during the Winter Session of 1865-66 at King’s College, London. Proc R Soc Lond 15:229–244.
Wozniak S, Pytrus T, Kobierzycki C, Grabowski K, Paulsen F. 2019. The large intestine from fetal period to adulthood and its impact on the course of colonoscopy. Ann Anat 224:17–22.
Yamamoto R, Izumida M, Sakuraya T, Emura K, Arakawa T. 2021. The ulnar nerve is surrounded by the tendon expansion of the flexor carpi ulnaris muscle at the wrist: an anatomical study of Guyon’s canal. Anat Sci Int 96(3):422–26.
You W, Henneberg R, Henneberg M. 2022. Healthcare services relaxing natural selection may contribute to increase of dementia incidence. Sci Rep 25;12(1):8873.
Yuan Z, Sunduimijid B, Xiang R, Behrendt R, Knight MI, Mason BA, Reich CM, Prowse-Wilkins C, Vander Jagt ChJ, Chamberlain AJ, MacLeod IM, Li F, Yue X, Daetwyler HD. 2021. Expression quantitative trait loci in sheep liver and muscle contribute to variations in meat traits. Genet Sel Evol 18;53(1):8.
Ziajkiewicz M, Kędzia A, Dudek K. 2010. Flexor carpi ulnaris (FCU) muscle (m. flexor carpi ulnaris) in foetal period. Arch Perinat Med 16(4):218–24.
Ziółkowski M, Trzaska M, Kurlej W, Porwolik K, Porwolik M. 1994. Relationship between the intervertebral foramina and the spinal nerves at the level of C4-T2 of the human fetal vertebral column. Folia Morphol (Warsz) 53(3):197–203.


Received: 15.11.2022; Revised: 13.12.2022; Accepted: 15.12.2022