Introduction
The index-finger and ring-finger ratio (2D:4D) is a retrospective biomarker that may indicate prenatal testosterone and estrogen exposure and have long-term effects on physiology, behaviour, fertility, disease risks, pubertal development, and reproductive success (Manning et al. 2000; Manning 2002; Matchock 2008; Manning and Fink 2011; Kalichman et al. 2013; Klimek et al. 2016; Kirchengast et al. 2020). The 2D:4D appears to be sexually dimorphic, with men typically having lower ratios than women due to earlier hormonal exposure to testosterone (Manning et al. 2000; Manning 2002; McIntyre 2006). A more masculine (low: 2D<4D) and more feminine (high; 2D>4D) digit ratios are clearly the result of increased prenatal testosterone and estrogen exposure, respectively (e.g. Manning et al. 1998; Klimek et al. 2016). Furthermore, 2D:4D may represent individual susceptibility to certain chronic diseases and hormonal disorders (Luijken et al. 2017; Tabachnik et al. 2020). The 2D:4D has been linked to higher levels of putatively androgenic outcomes, such as foetal and pubertal development and left-handedness (Manning et al. 2000; Manning 2002; Li et al. 2019), improved athletic ability, and an increased risk of autism (Manning et al. 2001; Mackus et al. 2017). It is also believed that the 2D:4D serves as a proxy indicator of intrauterine sex hormone levels because it significantly correlates with a number of somatic and behavioral characteristics as well as fertility measures (Manning et al. 1998; Fink et al. 2004; Klimek et al. 2016; Kirchengast et al. 2020). An explanation for the association between 2D:4D and prenatal sex hormone has been put forth as the action of the HOX A and HOX B genes, which regulate digit and toe differentiation and are implicated in sex determination, morphogenesis of the urino-genital system, appendicular skeleton, and fertility (Manning et al. 1998; Manning 2002; Manning et al. 2003; Zhang et al. 2013). The 2D:4D has also been connected to facial asymmetry (Fink et al. 2004), reproductive success and a longer reproductive period (Klimek et al. 2016), breast cancer (Muller et al. 2012), age at menarche (Matchock, 2008), age at menopause (Kirchengast et al. 2020), reproductive and general health (Tabachnik et al. 2020), and cardiovascular disease risk (Luijken et al. 2017). It has been recently reported that the 2D:4D ratio could predict an early or late onset of menarche (Matchock 2008; Manning and Fink 2011). Several studies have found that masculinized hands (low 2D<4D) are significantly related to delayed age at menarche (e.g., Matchock 2008; Manning and Fink 2011; Kalichman et al. 2013; Kirchengast et al. 2020). Therefore, more research is needed to ascertain whether there is a relationship between the 2D:4D and age at menarche in various populations in order to evaluate the population specific data on such a biological trait. The present study aims to determine the associations between the 2D:4D, which represents the prenatal hormonal environment (i.e., early androgen exposure) and the age at menarche of Sherpa tribal women living in Sikkim, India. Specifically, the aim of this study is to investigate the long-term influence of the prenatal hormonal environment as reflected in the 2D:4D on the onset of menarche in one of the indigenous populations in Sikkim, India.
Material and methods
The present community-based cross-sectional study was conducted among 119 Sherpa tribal women in the reproductive age group of 18–49 years in the Soreng district of Sikkim, North-East India. According to the 2011 National Census of India (2011), Sikkim had a population of 610,577 lakh (male: 323,070; female: 287,507) with a sex ratio of 890 (women to every 1000 men), which was lower than the national average of 940. Sikkim’s literacy rate was reported to be 81.42 percent (86.55% for men and 75.61% for women). Sherpa is an indigenous community in Sikkim that belongs to the Nepali ethnic group. According to the Denzong Sherpa Association, there are around 65,000 Sherpas in the population, with 32,000 of them speaking their mother tongue in their native environment. According to the 2011 National Census of India, a total of 13922 residents identified as Sherpa speakers in Sikkim. The Bodish or Bodic group organizes the Sino-Tibetan-Burman family, which includes the Sherpa language. The study participants were an ethnically homogeneous ethnic group of the Sherpa tribe of Sikkim, geographically situated in intermediate altitude locations (~7600 ft. and above). Study participants were selected using stratified random sampling methods. The Sherpa community was chosen for the study due to its vast distribution in the Soreng district of Sikkim. The homogeneity of the selected participants is determined in terms of their ethnicity, language, cultural practices, geographic distribution, history of ancestry, and shared beliefs in the community. The present study was conducted in the Sherpa-dominated village of Okhrey, located in the Soreng district of Sikkim, India. Okhrey village, with a population of 1,683 people, is mostly inhabited by Sherpa tribal populations and located in the Soreng subdivision of Sikkim’s west region (Male 821; Female 862). The covered community is located roughly 110 kilometres from Gangtok’s headquarters, the Daramdin BAC Block Administrative Center of Soreng district of Sikkim. The Soreng district is estimated to have 85,483 people (in 2021). According to the 2011 Indian census, this district has 64,760 residents (33,061 males and 31,699 women). Among the 44,921-literate people, there are 20,254 women and 24,667 men. Agriculture, the principal economy, employs 11,908 cultivators (4,171 women and 7,737 men respectively). A total of 1,949 individuals are estimated to work as agricultural labourers in Soreng, including 1,281 men and 668 women. The nearest town from Okhrey, about 20 km away, is Sombaria. A formula for calculating the sample size for a single percentage population was used to determine the estimated minimum sample size (n=96). With an anticipated proportion of 50% (p=0.5) based on the response distribution, a desired precision of 10% (E=margin of error) at the 95% confidence interval was used. Present study has used the following formula to calculate the sample size for a single proportion: n=Z2×p×(1−p)/E2. In total, a sample size of 119 Sherpa tribal women was collected for the present study. The fieldwork was completed between February and April 2022. Before collecting data, study participants who willingly participated in the study provided informed consent. The participants’ ages and ethnicities were verified using birth certificates and official documents. The participants’ average age was 27.81±8.65 years. The objectives and scope of the present study were explained to the participants. Pre-structured schedules and household survey methods were used to collect data on age at menarche, anthropometric measures, and demographic factors, which was subsequently used to calculate the respondents’ age at menarche. Study participants were asked to describe the entire year when they first experienced menstrual bleeding. The participation in this examination was entirely voluntary, and the overall response rate was relatively high, with all eligible persons who met any excluding criteria taking part in the present study. The present study was carried out in accordance with the Helsinki Declaration’s ethical guidelines for human research (Portaluppi et al. 2010).
Collection of Anthropometric Measurements
The standard methods for obtaining finger length measurements were used (Manning et al. 1998). A single experienced observer measured the finger lengths from the proximal crease at the base of the finger to the tip of the finger in the midline on the palmar aspect of the hand using a vernier calliper nearest to 0.1 mm without exerting pressure; protruding fingernails were excluded. The participant’s hand was placed on a plastic board, palm up, to obtain the digit lengths [i.e., index finger (2D) and ring finger (4D)]. This measurement has been reported to be highly repeatable (Manning et al. 1998). Participants with digit/hand abnormalities/fractures, injuries, or surgical episodes were excluded from participating in the study. However, the participants in this study were also measured precisely to avoid any possible systematic errors and to establish landmarks in the process of anthropometric data collection. The mean values of two consecutive measurements were reported. The mean differences in repeated measurements were found to be statistically insignificant (p>0.05). The technical errors of measurement TEM=(D2/2N), where “D” represents the difference between anthropometric measurements and “N” represents the number of individuals measured, is an accuracy index that evaluates the standard deviation between repeated measures used to determine the consistency of anthropometric measurements. The ideal approach involves calculating relative TEM followed by calculating the reliability coefficient [R=1-(TEM)2/SD2], (SD=the standard deviation of all measurements) (Goto and Mascie-Taylor 2007). The TEM was determined using numerous measurements taken from 30 randomly chosen Sherpa women. In TEM analysis, very high reliability (R) (R>0.975) was achieved in both left and right 2D and 4D lengths, and values were found to be within the permissible range of R=0.95 (Goto and Mascie-Taylor 2007).
Statistical Analysis
The Statistical Package for Social Sciences (SPSS; Version 16.0) was used to analyse the data. In descriptive statistics, continuous variables were represented by mean and standard deviation (SD), and the Kolmogorov-Smirnov (K-S) non-parametric test was used to assess whether the data distribution’s normality significantly deviated from the predetermined theoretical distribution. The length of the 2D was divided by the length of the 4D to calculate the 2D:4D digit ratio. Further, the study participants were divided into two groups based on the 2D:4D values for both hands: more feminine (2D:4D≥1) and more masculine (2D:4D<1), in accordance with previously described methodologies (e.g., Klimek et al. 2016). In particular, this study compared groups of reproductive women with more masculine (2D:4D<1) and more feminine (2D:4D≥1) characteristics to examine the relationship between 2D:4D and the age of menarche. A One-Way Analysis of Variance (ANOVA) was used to compare the category-wise means of the finger length variables and age at menarche. The linear association between two or more continuous variables, such as digit lengths, 2D:4D, and the age of menarche, was determined using Pearson’s correlation coefficient as well as linear and multiple regression models. A p-value < 0.05 was considered statistically significant.
Results
Table 1 depicts the descriptive statistics (mean ±SD) of 2D and 4D lengths and 2D:4D and age at menarche among Sherpa tribal women. Sherpa women had a mean menarche age of 13.17 (±1.23) years. The mean and SD for the right hand 2D:4D were 0.99±0.05, and the mean SD for the left hand 2D:4D was 0.99±0.06. The results of K-S non-parametric test indicate that age at menarche (K-S value: 0.23; p<0.01), left 2D:4D (K-S value: 0.11; p<0.01) and right 2D:4D (K-S value: 0.12; p<0.01) p-values are small, which show significant deviations from the normal distribution, thus indicate nonlinearity in the data distribution. The Boxplot and Q-Q plots of age at menarche, 2D:4D distribution, and deviation from normality of the data are depicted in Fig. 1. Using ANOVA, the mean differences in finger lengths, 2D:4D, and age of menarche were shown to be statistically significant between the feminine and masculine categories (p<0.01) (Table 1). A Pearson correlation analysis plot of the 2D and 4D lengths and 2D:4D with age at menarche among Sherpal reproductive women is presented in Fig. 2.
| Variables | Mean ±SD (N=119) | 95% CI of Mean | Left | Right | ||||
| Masculine (Low 2D:4D <1.00) (N=81) |
Feminine (High 2D:4D≥1.00) (N=38) |
F-value p-value |
Masculine (Low 2D:4D <1.00) (N=77) |
Feminine (High 2D:4D≥1.00) (N=42) |
F-value p-value |
|||
| 2DL mm | 61.96±4.32 | 61.18−62.75 | 60.91±4.25 | 64.23±3.60 | 17.15** | 61.51±4.61 | 62.79±3.65 | 2.43 |
| 2DR mm | 62.33±4.71 | 61.48−63.19 | 62.13±4.93 | 62.76±4.25 | 0.45 | 61.04±4.59 | 64.70±4.00 | 18.84* |
| 4DL mm | 62.81±4.31 | 62.03−63.60 | 63.45±4.59 | 61.46±3.28 | 5.78* | 62.89±4.51 | 62.68±3.94 | 0.06 |
| 4DR mm | 63.20±4.63 | 62.36−64.04 | 63.57±4.76 | 62.41±4.29 | 1.64* | 63.84±4.74 | 62.04±4.24 | 4.23* |
| 2D:4D (Left) | 0.99±0.05 | 0.98−1.00 | 0.96±0.03 | 1.05±0.03 | 184.71** | 0.98±0.05 | 1.00±0.06 | 6.78* |
| 2D:4D (Right) | 0.99±0.06 | 0.98−1.00 | 0.98±0.04 | 1.01±0.07 | 7.79** | 0.96±0.03 | 1.04±0.05 | 153.52** |
| Age at Menarche (years) | 13.17±1.23 | 12.94−13.39 | 13.36±1.23 | 12.75±1.16 | 6.66* | 13.36±1.27 | 12.82±1.14 | 5.26* |
*p<0.05;**p<0.01
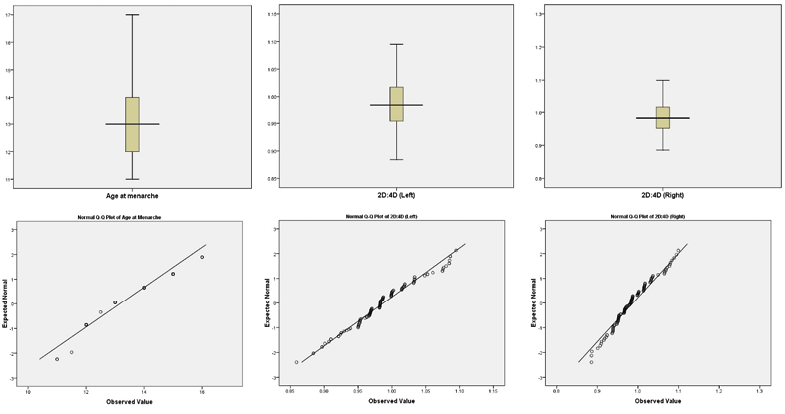
Fig. 1. The distribution and deviation of age at menarche, 2D:4D (left) and 2D:4D (right) using Box-plot and Q-Q plot among Sherpa tribal women of Sikkim, India
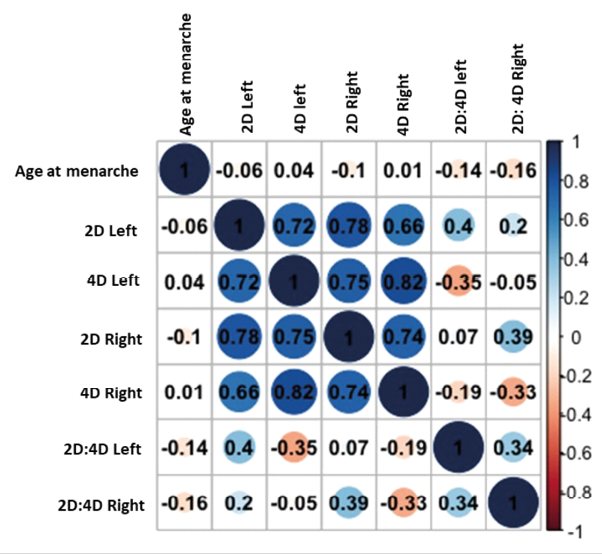
Fig. 2. Pearson correlation analysis plot of the 2D and 4D lengths and 2D:4D with age at menarche among Sherpa tribal women of Sikkim, India
Pearson correlation coefficient analysis revealed that age at menarche is significantly (p<0.01) and negatively correlated with the 2DL (r=−0.06) and 2DR (r=−0.10) but positively correlated with the 4DL (r=0.04) and 4DR (r=0.01) (p>0.05). A negative coefficient implies that a lower 2D:4D ratio is associated with an earlier age at menarche, whereas a positive coefficient indicates a later age at menarche. On the other hand, age at menarche was found to be inversely linked with 2D:4D left (r=−0.14) and 2D:4D right (r=−0.16) (Fig.2). Thus, the correlation analysis of 2D:4D does not show a strong and statistically significant relationship with the age at menarche (p>0.05).
The more masculine 2D:4D (<1.00) was found to be more frequent in both the left (63.70%) and right (61.30%) hands of participants, whereas only 36.52% (in the left hand) and 38.70% (in the right hand) demonstrated a more feminine 2D:4D (≥1.00). The category-wise mean comparison revealed that Sherpa women with a more feminine 2D:4D (≥1.00) had a substantially earlier attainment of menarche (in years) in both the left (12.75 vs. 13.36; p<0.05) and right (12.82 vs. 13.36; p<0.05) hands compared to those exhibiting a more masculine 2D:4D (<1.00). The linear and multiple regression analysis revealed that digit length (2DL, 2DR, 4DL, and 4DR) and 2D:4D left and right were strongly related to age at menarche (p<0.01). The regression coefficient for the age at menarche on 2D:4D left was 0.14 (t-value= 7.41, p<0.01), while the regression coefficient for age at menarche on 2D:4D right was 0.16 (t-value= 8.36; p<0.01), indicating that the right 2D:4D was more significantly associated with age at menarche (p<0.01). Multiple regression analysis for age at menarche on left and right 2D:4D was statistically significant (p<0.01) (Table 2). Regression analysis confirmed a significant association between 2D:4D and the age of menarche. Furthermore, 2D:4D appears to influence the timing of menarche in Sherpa women, with more masculine 2D:4D (<1.00) associated with later attainment of menarche.
| Variables | Linear Regression Model | R | R2 | SEE | t-value | p-value |
| 2DL (mm) | 14.246 −0.017 | 0.061 | 0.004 | 1.242 | 8.673 | <0.001 |
| 2DR (mm) | 14.885−0.028 | 0.105 | 0.011 | 1.237 | 9.884 | <0.001 |
| 4DL (mm) | 12.527 +0.010 | 0.035 | 0.001 | 1.243 | 7.483 | <0.001 |
| 4DR (mm) | 14.885−0.028 | 0.105 | 0.011 | 1.237 | 9.854 | <0.001 |
| 2D:4D (Left) | 16.459+3.333 | 0.136 | 0.018 | 1.232 | 7.410 | <0.001 |
| 2D:4D (Right) | 16.693−3.569 | 0.161 | 0.026 | 1.227 | 8.357 | <0.001 |
| 2DL+4DL (mm) | 13.377−0.051 (2DL) +0.047 (4DL) | 0.129 | 0.017 | 1.239 | 7.499 | <0.001 |
| 2DR+4DR (mm) | 13.933−0.065 (2DR) +0.052 (4DR) | 0.169 | 0.029 | 1.231 | 8.494 | <0.001 |
| 2D:4D (Left) + 2D:4D (Right) | 18.236 + 2.251 (2D:4D L) −2.881 (2D:4D R) | 0.183 | 0.033 | 1.228 | 7.069 | <0.001 |
Discussion
Over the last two decades it has been strongly suggested that 2D:4D is an indirect biomarker of intrauterine steroid levels, such as testosterone (Manning et al. 1998; Manning et al. 2000; Manning 2002; McIntyre 2006; de Sanctis et al. 2017). The present study advances our knowledge of the association between prenatal sex hormone exposure indicated by the 2D:4D ratio, and age at menarche among Sherpa tribal women in Sikkim, India (Table 1). The 2D:4D values have been used to predict reproductive capacity and success, fertility measures, natural menopause, and age at menarche (Manning 2002; Klimek et al. 2016; Kirchengast et al. 2020; Tabachnik et al. 2020). Furthermore, biological and cultural factors, such as age at menarche, age at marriage, breast feeding, nutrition, postpartum ovarian function, illness prevalence, early age at natural menopause, and reproductive period, have been found to have a considerable impact on population reproductive performance (Matchock 2008; Klimek et al. 2016; Kirchengast et al. 2020). This is interesting because numerous studies have discovered that women exhibiting a feminine 2D:4D (≥1.00) tend to marry at a younger age, prefer marriage at a younger age, have more successful pregnancies and children (Manning and Fink 2008; Klimek et al. 2016; Eresheim et al. 2020; Kirchengast et al. 2020) and experience menopause at a later age (Kirchengast et al. 2020). This study found that women with high 2D:4D (≥1.00) had marginally earlier age at menarche compared to those with low 2D:4D (1.00) (p>0.01) (Table 1). Women having a feminine 2D:4D (≥1.00) were more likely to reach menarche early than women with masculine 2D:4D (<1.00) (Matchock 2008; Manning and Fink 2011; Eresheim et al. 2020; Tabachnik et al. 2020).
Several researchers have hypothesized that prenatal testosterone levels and 2D:4D are negatively correlated (Manning et al. 1998, 2000, 2003; de Sanctis et al. 2017). The possible mechanisms for this correlation are supported in part by lower 2D:4D with congenital adrenal hyperplasia and higher 2D:4D with complete androgen insensitivity syndrome, as well as a relationship between 2D:4D and polymorphisms in the androgen receptor. However, a few contradictory findings has also been reported in order to establish a possible mechanism linking 2D:4D or testosterone secretion reduction with genetic disorders, such as Klinefelter syndrome (Manning et al. 2013), offspring birth sex ratio (Helle and Lilley 2008), age at menarche (Helle 2010; Muller et al. 2012), and salivary testosterone change under acute exercise (Kowal et al. 2020). For instance, Helle (2010) revealed no indication that the 2D:4D of the right or left hand is related to menarche age in Finnish women while Gooding and Chambers (2018) identified no statistically significant link between digit ratios and menarche age in women (p>0.05).
Several studies have shown that the 2D:4D is strongly and inversely related to menarcheal age in the population (Matchock 2008; Manning and Fink 2011; Oberg and Villamor 2012; Kalichman et al. 2013; Tabachnik et al. 2020; Eresheim et al. 2020). Research on the relationship between 2D:4D and age at menarche (Manning et al. 2003; McIntyre 2006; Matchock 2008) supports a delayed menarcheal association with more androgen exposure during the intrauterine period. Hence, greater exposure to androgens in the womb is thought to have an impact on the timing of pubertal development, including the onset of menarche. In addition, women who delay menarche may have a less than ideal reproductive profile, which increases their risk of sub-fecundity and infertility (Guldbrandsen et al. 2014; Tabachnik et al. 2020). According to several studies, early menarche is associated with a higher risk of diseases like cancer, type 2 diabetes, hypertension, cardiovascular disease, and metabolic disorders manifesting later in life (Muller et al. 2012; Luijken et al. 2017; Wang et al. 2018). The present study also shows a significant negative correlation between the left hand 2D:4D and menarche age. Some researchers have proposed that the left 2D:4D is a reliable indicator of prenatal hormone exposure, and that the left side of the body may significantly reflect female-typical factors, puberty traits and development, and the prevalence of specific diseases (Manning 2002; Wang et al. 2018; Li et al. 2019).
The results of the present study showed that left 2D:4D was significantly inversely related to women’s age at menarche. Several studies have reported the associations between left hand 2D:4D and biological factors, habits, and disease in females (e.g., Mackus et al. 2017; Wang et al. 2018). It is believed that the left-hand 2D:4D is a useful predictor of a variety of reproductive features, such as age of menarche and reproductive success and thus it is biologically appropriate for reproductive women to examine the 2D:4D in both hands (Manning and Fink 2008; Klimek et al. 2016; Tabachnik et al. 2020). However, Manning and Fink (2011) reported a negative relationship between right 2D:4D and age of menarche in a large population group of women. Overall, while the present study adds to our understanding of the association between 2D:4D and menarche age, its limitations must be mentioned when extrapolating its results to other populations or contexts. To further understand the association between the 2D:4D and age at menarche in adults, future research might benefit from larger sample sizes, longitudinal designs, and comprehensive confounding factor control.
Overall, the results of this study showed that a low 2D:4D as a proxy measure for prenatal hormone exposure may be associated with delayed menarche and therefore reproduction in women. This study also shows that prenatal hormonal exposure, indicated by the 2D:4D, is a significant factor determining age at menarche among Sherpa tribal women; hence, women with high prenatal testosterone and low prenatal estrogen tend to have delayed menarche.
Acknowledgement
The authors are gratefully acknowledged the help and cooperation of village level authorities and research participants of the present study. The extended help and cooperation of the Department of Anthropology, Sikkim University is also being acknowledged.
Conflict of interest
The authors do not have conflict of interest to declare.
Authors’ contribution
NM: Conceived the idea, carried out the literature search and analysed the data, and wrote the manuscript. RR: Conducted the fieldwork and collected the data, carefully revised the draft manuscript, and contributed important intellectual content to the final manuscript. All the listed authors have contributed substantially to finalisation of the manuscript.
Funding
The financial assistance in the form of University Grants Commission-Non-NET Fellowship to Rebaka Rai is also being acknowledged
* Corresponding author: Dr. Nitish Mondal, Ph.D., Associate Professor, Department of Anthropology, Sikkim University, Gangtok 737102, Sikkim, India, e-mail: nmondal@cus.ac.in
 https://orcid.org/0000-0001-5698-4430
https://orcid.org/0000-0001-5698-4430

