Available online at: https://doi.org/10.18778/1898-6773.85.4.07
Division of Anthropology, Institute of Environmental Biology, Wrocław University of Environmental and Life Sciences, Wrocław, Poland
Human Anatomy Department, J.M. Vargas Medical School, Faculty of Medicine, Universidad Central de Venezuela, Caracas, Venezuela
ABSTRACT: Wormian bones (WB) are the irregular bone structures developed from additional centers of ossification. Although they are commonly found in healthy individuals, under certain conditions (number >10, mosaic pattern, large size), they can indicate pathology. While their coexistence with numerous diseases is well-documented, and various studies have reported their prevalence in populations of various geographic regions, no qualitative critical review of such studies has been conducted. The aim of this paper is to perform a critical review of research studies on the presence of Wormian bones in populations worldwide, with a particular emphasis on the methodology used and the selection of the samples studied.
A sample of 44 original research articles was selected via PubMed and Google Scholar databases. Four criteria were assessed: 1) number of individuals in each group, 2) known sex of individuals, 3) selection criteria of individuals, and 4) implementation of the statistical analysis. The origin of the research sample was determined as well as the method of the WB calculation, and data on the WB prevalence worldwide was collected in tabular form.
The reported size of the research samples varies from 22 to 628 individuals, derived from both contemporary and archaeological populations. Four major formulas were used in order to provide the frequency of WB. The sex of individuals was known in 18 (40.9%) articles. Most of the articles focused on Asian samples.
The difficulties in comparing data on the Wormian bones are caused by considerable inconsistency in the methodology used to research this phenomenon. Therefore, the interpopulation comparisons currently made may not be correctly estimated. Our study highlights the need for using more comprehensive and consistent data collection as well as processing protocol suitable for populational research on sutural bones.
KEY WORDS: cranium, skull, suture, sutural bone, Wormian bone
The human skull consists of two developmentally and functionally distinct groups of bones: viscerocranium and neurocranium (Reicher and Łasiński 2010). The number of skull bones is generally constant, but in some cases, the number might increase due to the presence of so-called Wormian bones (WB) which are also known as ‘intrasutural bones’, ‘sutural bones’, ‘skull accessory ossicles’, ‘supernumerary ossicles’, ‘intercalated bones’, ‘accidental bones’, ‘intercalary ossicles’, ‘Schaltknochen’, ‘ossa Wormiana’, ‘ossa suturalia’ (Goyal et al. 2019; Nowak et al. 2018; Rajni et al. 2018; Murlimanju et al. 2011). In particular cases – when the bones occur in the position fontanelles (Natsis et al. 2019; Nikolova et al. 2016) – they have specific names such as ‘bregmatic bone’ and ‘Kerckring bone’ (in bregma, Nikolova et al. 2016; Vishali et al. 2012), or ‘pterion ossicles’ and ‘epipteric bone’ (in pterion, Standring 2016). There is also a large bone on the posterior part of the skull (upper area of the occipital squama) referred to as the ‘Inca bone’ (Cirpan et al. 2014) or ‘interparietal bone’.
Wormian bones are irregular, diversiform bone structures developed from the additional centers of ossification (Nowak et al. 2018; Bellary et al. 2013) and interjected in the cranial sutures. They are present not only in modern humans but also in the archeological context (Nikolova et al. 2014; Panzer et al. 2014), in Neanderthals (Bruner 2004), and animals (Zambrano et al. 2021; Smith et al. 1977).
The size of a single accessory bone can vary from less than 1 mm x 1mm up to more than 5 cm x 9 cm in width and length (Sreekanth and Samala 2016). In addition, these bones have multiple shapes: oval, round, oblong, quadrilateral, and irregular (Parker 1905, as cited in Murlimanju et al. 2011). Wormian bones are generally a feature of the neurocranium and occur mostly in the lambdoid suture, especially on the right side (Nowak et al. 2018; Bellary et al. 2013). Other frequent localizations are coronal suture, bregma, lambda, pterion, and asterion. However, WB may, although rarely, occur in the sutures connecting the craniofacial bones – for example in the frontonasal suture (Edwards et al. 2017) or in the orbital cavity between the frontal, lacrimal and ethmoid bone (Rizvi et al. 2018). The anatomical denomination of Wormian bones is still debated (Romero-Reverón 2020) while the etiology of sutural bone formation still remains unclear. It has been hypothetised that WB might indicate genetic factors (Finkel 1975; Bennett 1965), mechanical pressure on the skull bones in early stages of ontogenetic development, such as artificial cranial deformations (Sanchez-Lara et al. 2007; O’Loughlin 2004; El-Najjar and Dawson 1977), metabolic disorders (Hess 1946, as cited in Jeanty et al. 2000), as well as environmental factors (Barberini et al. 2008; Sanchez-Lara et al. 2007). Currently, more holistic interpretations are adopted that recognize the influence of all the aforementioned factors in WB development (Di Ieva et al. 2013; Barberini et al. 2008). Some studies suggest that the presence of sutural bones might indicate developmental instability (Di Ieva et al. 2013; Vishali et al. 2012; Barberini et al. 2008).
The Wormian bones have been reported to commonly occur in healthy populations, and their presence typically is not associated with any pathological conditions (Natsis et al. 2019; Andrade et al. 2018; Johal et al. 2017; Walulkar et al. 2012); however, their significant number (above 10), size (more than 6 mm x 4 mm), or characteristic mosaic pattern are clinically considered as indicators of several congenital diseases, mostly osteogenesis imperfecta (Cremin et al. 1982) exhibited by abnormally numerous Wormian bones (Semler et al. 2010). Other diseases are also frequently associated with WB, such as hypophosphatasia, craniosynostosis, hypothyroidism, cleidocranial dysostosis, rickets, pyknodysostosis (osteopetrosis acro-osteolytica), pachydermoperiostosis, congenital hypothyroidism, hydrocephalus, otopalatodigital syndrome, Hajdu-Cheney syndrome, Menkes syndrome (Ratnaningrum 2020; Saylisoy 2020; Basnet et al. 2019; Romero-Reverón and Arráez-Aybar 2019; Kumar and Ratna Prabha 2016; Marti et al. 2013). Many of them are congenital disorders or CNS (central nervous system) anomalies. Studies have shown that Wormian bones can help in diagnosis and treatment of those conditions (Goyal et al. 2019; Romero-Reverón and Arráez-Aybar 2019; Jeanty et al. 2000). As Wormian bones are developed prenatally (Jeanty et al. 2000), they can be detected during the routine USG examination, and used as the prenatal diagnosis of severe or lethal conditions (Tonni et al. 2013).
Wormian bones can be also considered as important from the medicolegal perspective as their characteristic pattern, can be used for identification of an individual (Kuharić et al. 2011; Jayprakash 1997); moreover, due to their association with abnormal bone brittleness, WB can prove critical in the diagnosis of the children’s bone trauma in order to exclude the possibility of physical abuse (Johal et al. 2017; Govsa et al. 2014; Marti et al. 2013). During X-Ray examination of the head, Wormian bones can also be mistaken for fractures (Narayan et al. 2019; Romero-Reverón 2017). Radiologists and surgeons have been advised to take WB into account while planning surgical interventions to the head to avoid possible injury (Ratnaningrum 2020; Kumar and Ratna Prabha 2016).
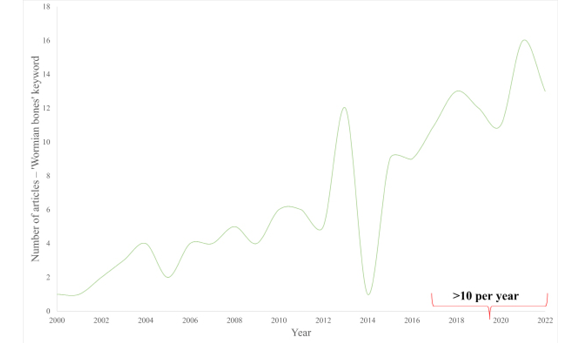
Interest in Wormian bones in the last decade has been significantly growing (see fig. 1).

Fig. 1. Number of articles published between 2000 and 2022, found in PubMed by the ‘Wormian bones’ keyword (full texts only)
Some studies have discussed the association of WB with various diseases (case reports), while other have established the relationship between WB and biological phenomena, such as metopism (Li et al. 2022; Cirpan et al. 2016), individual genes or groups of genes that influence WB formation (Zimmerman et al. 2019; Kague et al. 2016), and variants of the skull morphology (Basnet et al. 2019). Since WB are considered as an anthropological marker of the interpopulation distance (Natsis et al. 2018; Gümüsburun et al. 1997; Pal and Routal 1986), some studies report the WB prevalence in human populations worldwide. In many cases, the data presented and juxtaposed therein are further cited by researchers; however, so far, no qualitative critical analysis of this group of articles has been carried out. Such a comprehensive review of the above studies, however, would be very useful for other researchers by providing an established basis for future research on Wormian bones.
The aim of this study is to review articles on the presence of Wormian bones in populations worldwide, with a particular emphasis on the methodology used and the selection of the study sample. In this study, the term ‘Wormian bones’ is used predominantly due to its common occurrence in the reviewed articles.
This study is a semiqualitative review of literature. By using ‘Wormian bones’ as the keyword search term, 208 articles in the PubMed database were found. After adding the filter for ‘full text’, the total number was 169 (August 22, 2022).
In addition, in order to supplement the study material, the authors also used the Google Scholar database. Overall, the number of records found using the same ‘Wormian bones’ search term was 5870 (citations and patents were not taken into account). To limit the scope of the material, the following search criteria were used:
The total sum of records found using the above search criteria was 2600 (August 22, 2022). Since the search with the ‘highest relevance’ option was used, the further review was limited to the first five pages of Google Scholar results (50 articles), considering them to be the most relevant to the search criteria.
The total number of records obtained at this stage of search (preliminary database) was 219.
At the following stage, the articles were reviewed in terms of meeting the following selection criteria:
After excluding duplicates (publications recorded both in PubMed and Google Scholar databases) the total number of articles was 176.
Such obtained material was then divided into six groups/categories: 1) case reports, 2) original articles on clinical approaches, 3) medicolegal approaches, 4) original articles on Wormian bones prevalence in populations worldwide, 5) reviews, 6) varia (i.e., articles directly or indirectly related to Wormian bones, which were not assigned to any of the above category). Only the fourth group (original articles on Wormian bones prevalence in populations worldwide) was selected as the subject of this research. An article was assigned to this group if it indicated the studied population in the title, introduction, or in the description of the research material. Articles that investigated the relationship between WB and skull deformities in various populations were excluded from the study. The preliminary number of articles included into the fourth group was 39.
In order to enrich the material, the authors checked the reference lists of these articles and selected additional publications regarding the WB prevalence in particular populations that were not included during the previous selection steps. Although such identified articles were only few, they report relevant results that are cited in further research as reference data. Because this study is, to our knowledge, the first attempt to propose a semiqualitative review of the WB issue, the chosen articles were not selected for peer review, citation score, impact factor, or other parameters. The final number of articles in the fourth group was 44 (see fig. 2), published between 1975 and 2022.
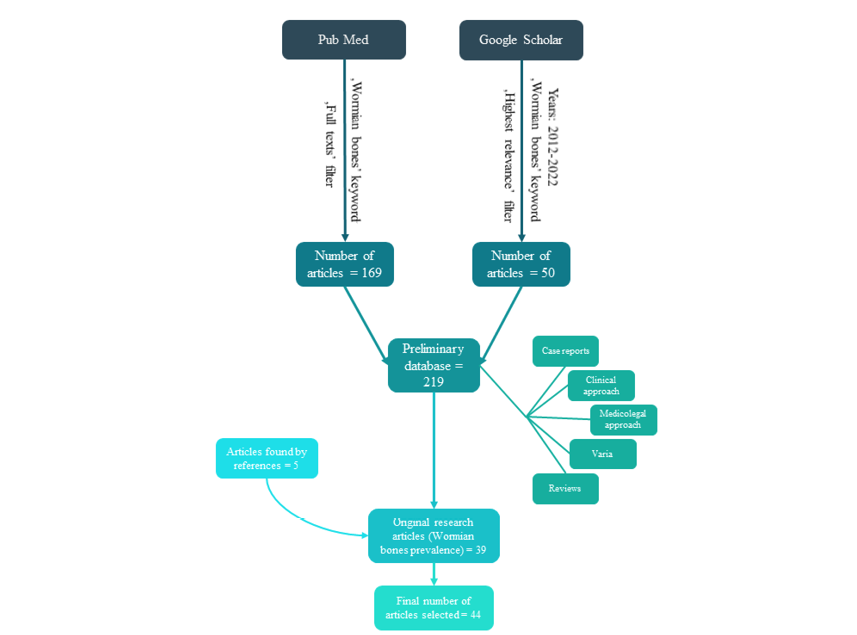
Fig. 2. Articles selection process
A scoring system, inspired in part by the Bradford-Hill criteria (Rothman and Greenland 2005), was proposed for the assessment of the evidential strength of the analyzed articles. Four benchmarks were selected, and one point was noted for each criterion:
The data was tabulated according to the point category: 0–4 points. Central tendency measures were calculated using the STATISTICA 13.3 software (TIBCO; no. of license: JPZ007B482801ARACD-9).
Due to the scoring system implemented and discussed above, the division of the articles from the group of articles selected for review is as follows: 0 points – 1 article; 1 point – 11 articles; 2 points – 15 articles; 3 points – 12 articles; 4 points – 5 articles (table 1). The reported size of research samples varies from 22 to 628 individuals (mean = 125, Me = 79, Mo = 50, Q1 = 50, Q3 = 155.5). Research samples used in these studies were collected from contemporary institutions (e.g., post-sectional dry skulls, exhibits of medical museums with the number of articles of 36), or historical populations (e.g., from archaeological excavations or museums with a number of 5 articles), or mixed sources with only one article. In a few cases the origin of samples was unclear. Where possible, the authors determined the origin of the sample on the basis of the condition of the skulls visible in the photographs. In 18 studies (40.9%), the sex of the subjects was known. The age of individuals was known in 33 (75%) of all examined articles, although in most cases they were described simply as ‘adults’. In two articles, the division of individuals into age categories was given as follows:
Some articles were thematically limited to selected points of the skull: asterion – 3 articles; – pterion – 7 articles; asterion and pterion – 1 article; preinterparietal/interparietal/Inca bones – 4 articles. In two cases, the preinterparietal/interparietal/Inca bones were excluded from the study.
The described research was conducted mainly in the area of South Asia (see fig. 3) with a high representation of data from India.
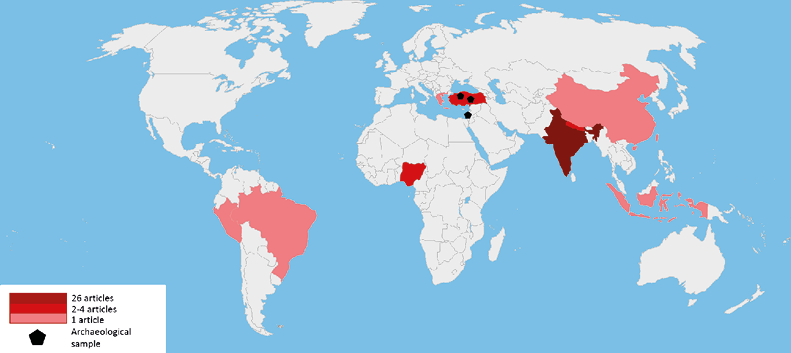
Fig. 3. Geographical distribution of research on Wormian bones (blank map by Ian Maphy, the authors’ modification)
In 24 out of 44 articles (54.5%), a value of general prevalence of Wormian bones in a particular population was reported. This value varied from 4.7% in Rajasthan (Masih et al. 2013) to 88.8% in South India (Nayak and Shetty 2019). Moreover, the articles provide the WB frequency in particular skull sutures (table 2) revealing that the site of the most frequent occurrence of these structures was the lambdoid suture, which corresponds to other studies (El-Najjar and Dawson 1977; Romero Reverón 2017). The next most common locations for sutural bones were the asterion (79.7% in South Indian population, Lekshmy et al. 2017), coronal suture (66.6% in South Indian population, Nayak and Shetty 2019), and the squamous suture (45.5%, also in South Indian population, Lekshmy et al. 2017) – the highest percentages recorded are given in this study. The prevalence of preinterparietal or interparietal or Inca bones occurrence varies from 1.3% in Central India (Marathe et al. 2010) to 13.3% in South India (Nagarajan and Ganesh 2017). All percentages listed indicate the number of individuals with a Wormian bone in specific cranial site (suture), relative to the entire study group.
26 articles (59.1% of reviewed sample) relate to the Indian population. Articles investigating populations from other areas of Asia taken into account included Nepal = 2, Indonesia = 1, Turkey (including Anatolia) = 6, China = 1, and Israel (Lachish) = 1. Data on Asian populations (including archaeological) account for over 84% of the sample. The remaining articles concern Greek (1 article), Nigerian (4 articles), Brazilian (1 article), Peruvian (1 article).
The assessment of the variability in the occurrence of biological phenomena in a population is the first and indispensable step towards analyzing the etiology of any given phenomenon. The collected and available data on the variability of the phenomenon in other populations allow for comparative analyzes. Researchers investigating Wormian bone in the analyzed populations often refer to tabulated data from other studies (Li et al. 2022; Sah et al. 2017; Kalthur et al. 2017; Ghosh et al. 2017; Gümüsburun et al. 1997). In the above studies, the researchers refer to the global ranges of variability determined by the populations with the lowest and highest percentage of sutural bones – such as: “The reported incidence is variable, ranging from around 10% (in Caucasian skulls), through 40% (in Indian skulls), to 80% (in Chinese skulls)” (Khan et al. 2011).
This study reviews the original articles that reported the incidence of Wormian bones in populations from different geographic regions in order to create an organized database; however, the difficulty in completing such a database results from the inconsistency of the methods used to assess the presence of the Wormian worldwide. The most considerable differences in the adopted methodology concern calculating the frequency (percentage) of a feature in the analyzed publications. Four dominant strategies were shown (table 2):
In 5 cases, either a different way of calculating attendance was used or it was not specified how the result was obtained. The inconsistency of the calculation methods in which the result is reduced to one value category – the percentage (%) – creates a risk that the data obtained and compiled with each other may be under- or overestimated. Inaccuracies also apply to the classification of the Wormian bones themselves; for example, (pre-)interparietal/Inca bones are treated as a separate category or included into the group of lambda bones or sagittal/lambdoid sutures.
Of the selected studies, 2 were conducted on archaeological samples: Lachish (Finkel 1975) and Anatolian (Gümüsburun et al. 1997). These two studies are relatively often quoted in tabular summaries, in which they are compared with contemporary samples (Li et al. 2022). However, this approach may raise concerns because genetic distances between historical populations may differ considerably compared to those derived from contemporary populations. Moreover, a direct comparison of modern trials with groups that are significantly distant in time, such as the Lachish population analyzed in the article by Finkel (1975) dated as the late bronze age, may omit some aspects of morphological changes related to the process of human microevolution. For this reason, a greater precision in the description of the tested sample is important. One of the included in this review articles focused on the occurrence of morphological variants of pterion in mixed archaeological (13-century Byzantine) and contemporary (20-century Turkish) sample (Illknur et al. 2009). This study states that the pteric bone occurs in 6.25% od Byzantine sample and in 3.6% of the modern population. However, the statistical significance of differences in these both frequencies was not tested, and the samples were relatively small (28 modern skulls and 16 Byzantine skulls), so the obtained results may be unreliable (Illknur et al. 2009).
In 16 studies included in this review, the origin of the sample was not specified. It is also worth noting is that in some studies osteological materials from anatomical museums at medical faculties were used. The origin of such materials should also be carefully explained because museums can store both prepared contemporary remains and historical bone material (i.e., those from archaeological excavations including modern, medieval, and even older remains) in their collections.
Modern samples may not only differ from archaeological samples in terms of genetic distance, but also be expoused to different environmental conditions, exhibit different health status which may, potentially, affect the incidence of Wormian bones and the high number of which is associated with numerous diseases. Furthermore, historical material from archaeological excavations or crypts is subject to taphonomic changes. For example, an excavated material may be damaged or dehydrated in such a way as to posthumously unseal the space of the cranial sutures. This poses a risk of accidentally losing Wormian bone post mortem. Such a risk is much smaller in comparison with contemporary post-sectional samples. In historical populations, the structure of sex and age is also different, which may distort the interpretation of data.
In 26 out of 44 (59.1%) analyzed articles, the researchers did not specify the sex of the examined remains. The omission of such information makes it difficult to undertake further works on the study of sexual dimorphism, on which there is no consensus in the literature (Natsis et al. 2019; Cirpan et al. 2016; Patil and Sheelavant 2012). Some authors argue that there are no differences in the prevalence of WB between sex groups. However, according to the 16 discussed articles in which the sex of individual was determined (research on male group only excluded), it may appear that the Wormian bones are more common in males (table 3). Note, however, that these results may represent non-statistical observations, not based on a significant difference testing result.
In the four included in this review articles (4 of the 44, 9.1%), the examined sample consisted less than 30 individuals, which are considered minimum that allows inference on quantitative data. None of these studies attempted to estimate the required sample size. The collection of research material depends on many substantive factors, such as the total amount of osteological material obtained through archaeological excavations, non-substantive factors such as funds, consent to share the material, and the duration of the study. In population studies, providing a sample size that allows for statistical analyzes, although critical, was implemented only in 23 out of the 44 (52.3%) evaluated articles. Statistical inference with respect to compiling numerical data from population studies is the only way to distinguish significant biological relationships from numerical randomness. Without which it is rather difficult, if not impossible, to properly estimate the differences in the occurrence of Wormian bones between sex groups.
Of the 44 original articles included in the analysis, more than a half (26 of the 44, 59.1%) relate to the Indian population. Nevertheless, there is no consensus on the reported frequency of suture bones in populations from the same areas; consider the following examples: in the East Indies reported from 29.7% (Yadav and Salam 2020) to 72.3% (Purohit and Yadav 2019), in North India – from 35.3% (Goyal et al. 2019) to 51% (Rajni et al. 2018). These discrepancies may result from the large diversity of the populations inhabiting the area of India but also from the methodology adopted in the assessment of the Wormian bones.
Studies regarding populations from Asia account for over 84% of the sample collected according to the proposed method partly based on indications and systematic reviews of publications in established scientific databases. In contrast, there is an insufficient number of studies on populations from Europe, Africa, Australia, Oceania as well as North and South America. The problem of older research on WB, published in print (not digitized) in languages other than those used internationally, also should be mentioned.The lack of digital and linguistic access for researchers who want to undertake reviews or comparative analyzes can create considerable gaps in the methodology and results. An example of such materials could be the publication of Česnys and Balčiūnienė (1988) on Lithuanian populations; therefore, it is important to enrich available databases of scientific articles with archival works on the subject. Translated non-English-language studies and English-language meta-analyzes published in native languages concerning the prevalence of Wormian bones, as well as other morphological and anatomical studies in local populations, should be made available to the worldwide scientific and scholarly community.
Due to the major inconsistency regarding research methods used to determine the frequency of Wormian bones, comparing archival data may not be reliable and therefore existing knowledge regarding this phenomenon may not reflect the actual biological regularities. Consequently, there is a need for clarity regarding the description of the data collection protocol which would allow conducting a more robust meta-analysis and further research on Wormian bones. In addition, there is a significant gap in the data on the presence of sutural bones in non-Asian populations which should encourage research on materials from these regions of the world.
Acknowledgments
This work was supported by the Wrocław University of Environmental and Life Sciences (Poland) as the Ph.D. research program “Bon Doktoranta UPWr” no. N020/0006/20.
Conflict of interests
The authors declare no conflict of interests
Authors’ contributions
AB is the head of the research team, originator, main researcher, author of the working and final version of the paper, performer of statistical analyzes and interpretation of calculation results. RR-R is the co-creator of the draft and final version of the work and the substantive supervisor.
*Corresponding author: Agata Bisiecka, Kożuchowska Street 5, 51-631 Wrocław, Poland; e-mail: agata.bisiecka@upwr.edu.pl
Ahad M, Thenmozhi MS. 2015. Study on Asterion and Presence of Sutural Bones in South Indian Dry Skull. J Pharm Sci and Res 7(6):390–92.
Andrade LS, Kalthur SG. 2018. Topography of Wormian Bones in Cadaveric Dry Skulls. The Online Journal of Health and Allied Sciences 17(3):6. Available at https://www.ojhas.org/issue67/2018-3-6.html [Accessed 14 December 2022].
Barberini F, Bruner E, Cartolari R, Franchitto G, Heyn R, Ricci F, Manzi G. 2008. An unusually-wide human bregmatic Wormian bone: anatomy, tomographic description, and possible significance. Surg Radiol Anat 30(8):683–87, https://doi.org/10.1007/s00276-008-0371-0
Basnet LM, Shrestha S, Sapkota S. 2019. Prevalence of Wormian bones in dried adult human skulls: an osteo-morphometric study in Nepal. Anat Sci Int 94:101–09, https://doi.org/10.1007/s12565-018-0454-x
Bellary SS, Steinberg A, Mirzayan N, Shirak M, Tubbs RS., Cohen-Gadol AA, Loukas M. 2013. Wormian bones: A review. Clin Anat 26:922–27, https://doi.org/10.1002/ca.22262
Bennett KA. 1965. The Etiology and Genetics of Wormian Bones. Am J Phys Anthropol 23(3):255–60, https://doi.org/10.1002/ajpa.1330230313
Bruner E. 2004. Geometric morphometrics and paleoneurology: brain shape evolution in the genus Homo. J Hum Evol 47:279e303, https://doi.org/10.1016/j.jhevol.2004.03.009
Cirpan S, Aksu F, Mas N. 2014. Inca Bone in Human Skulls of the West Anatolian Populatıon. Int J Morphol 32(1):275–78, https://doi.org/10.4067/S0717-95022014000100045
Cirpan S, Aksu F, Mas N, Magden AO. 2016. Coexistence of Wormian Bones with Metopism, and Vice Versa, in Adult Skulls. J Craniofac Surg 27(2):493–95, https://doi.org/10.1097/SCS.0000000000002370
Cremin B, Goodman H, Spranger J, Beighton P. 1982. Wormian Bones in Osteogenesis Imperfecta and Other Disorders. Skeletal Radiol 8:35–38, https://doi.org/10.1007/BF00361366
De Lucena JD, Freitas FOR, Limeira ÍS, de Araújo Sales TH, Sanders JVS, Cavalcante JB, Cerqueira GS. 2019. Incidence of sutural bones at asterion in dry human skulls in Northeast Brazil, Acta Sci Anat.1(3):178–83.
Česnys G, Balčiūnienė I. 1988. Senųjų Lietuvos gyventojų antropologija. Vilnius, Lithuania: Mokslas. 154–160.
Durge SV. 2016. Study of Wormian Bones on Dry human skull and its sexual dimorphism in the region of Andhra Pradesh. IP Indian J Anat Surg Head Neck Brain 2(3):79–82.
Durgesh V, Rani CHR, Vijayalakshmi K, Khin Myo Thu, Venugopala Rao B, Viswakanth B. 2015. Incidence of Wormian Bones in the North Coastal Andhra Pradesh. IOSR-JDMS 14(10):53–57, https://doi.org/10.9790/0853-141085357
Di Ieva A, Bruner E, Davidson J, Pisano P, Haider T, Stone SS, Cusimano MD, Tschabitscher M, Grizzi F. 2013. Cranial sutures: a multidisciplinary review. Childs Nerv Sys 29:893–905, https://doi.org/10.1007/s00381-013-2061-4
Eboh DEO, Obaroefe M. 2014. Morphometric Study of Pterion in Dry Human Skull Bones of Nigerians, Int J Morphol 32(1):208–13, https://doi.org/10.4067/S0717-95022014000100035
Edwards B, Wang JMH, Iwanaga J, Luviano J, Loukas M, Oskouian RJ, Tubbs RS. 2017. Hiding Within the Cracks: Case Report of Rare Sutural Bone Found at the Nasion. Cureus 9(6):e1333, https://doi.org/10.7759/cureus.1333
El-Najjar M, Dawson GL. 1977. The Effect of Artificial Cranial Deformation on the Incidence of Wormian Bones in the Lambdoidal Suture. Am J Phys Anthropol 46:155–60, https://doi.org/10.1002/ajpa.1330460119
Finkel DJ. 1975. Wormian Bone Formation in the Skeletal Population from Lachish. J Hum Evol 5(3):291–95, https://doi.org/10.1016/0047-2484(76)90032-4
Ghosh SK, Biswas S, Sharma S, Chakraborty S. 2017. An anatomical study of Wormian bones from the eastern part of India: is genetic influence a primary determinant of their morphogenesis? Anat Sci Int 92(3):373–82, https://doi.org/10.1007/s12565-016-0342-1
Govsa F, Ozer MA, Bayraktaroglu S, Aktas EO. 2014. Anatomoradiological Identification of Intrasutural Bones for Importance of Cranial Fracture. Turkish Neurosurgery 24(3):357–62, https://doi.org/10.5137/1019-5149.JTN.8380-13.2
Goyal N, Garg A, Kumar Y. 2019. Incidence and Medicolegal Significance of Wormian Bones in Human Skulls in North India Region. Int J Appl Basic Med Res 9(3):165–68, https://doi.org/10.4103/ijabmr.IJABMR_89_19
Gümüsburun E, Sevim A, Katkici U, Adigüzel E, Güleç E. 1997. A study of sutural bones in Anatolian-Ottoman Skulls. Int J Anthropol 12(2):43–48, https://doi.org/10.1007/BF02447895
Illknur A, Mustafa KI, Sinan B. 2009. A Comparative Study of Variation of the Pterion of Human Skulls from 13th and 20th Century Anatolia. Int J Morphol 27(4):1291–98.
Jeanty P, Silva SR, Turner C. 2000. Prenatal diagnosis of Wormian bones. J Ultrasound Med 19(12):863–69, https://doi.org/10.7863/jum.2000.19.12.863
Jayprakash PT. 1997. Skull sutures: radiographic contour of Wormian bone as an individualising epigenetic marker. Can Soc Forensic Sci 30(2):39–47, https://doi.org/10.1080/00085030.1997.10757085
Johal J, Iwanaga J, Loukas M, Tubbs RS. 2017. Anterior Fontanelle Wormian Bone/ Fontanellar Bone: A Review of this Rare Anomaly with Case Illustration. Cureus 9(7):e1443, https://doi.org/10.7759/cureus.1443
Kague E, Roy P, Asselin G, Hu G, Simonet J, Stanley A, Albertson C, Fisher S. 2016. Osterix/Sp7 limits cranial bone initiation sites and is required for formation of sutures. Dev Biol 413(2):160–72, https://doi.org/10.1016/j.ydbio.2016.03.011
Kalthur SG, Vangara SV, Kiruba L, Dsouza AS, Gupta C. 2017. Metrical and non-metrical study of the pterion in South Indian adult dry skulls with notes on its clinical importance. Marmara Med J 30:30–39, https://doi.org/10.5472/marumj.299387
Khan AA, Asari MA, Hassan A. 2011. Unusual presence of Wormian (sutural) bones in human skulls. Folia Morphol 70(4):291–294.
Kuharić J, Kovacic N, Marusic P, Marusic A, Petrovecki V. 2011. Positive Identification by a Skull with Multiple Epigenetic Traits and Abnormal Structure of the Neurocranium, Viscerocranium, and the Skeleton. JFS 56(3):788–93, https://doi.org/10.1111/j.1556-4029.2011.01718.x
Kumar U, Ratna Prabha J. 2016. Wormian bones: study on dry human skulls in North Karnataka region. IJAR 4(1):1854–58, https://doi.org/10.16965/ijar.2015.351
Lekshmy VVG, Ramakrishna A, Meera J. 2017. Incidence of Wormian bones in dry human skulls in South Indian population. Int J Anat Res 5(3.3):4349–55, https://doi.org/10.16965/ijar.2017.331
Li J-H, Chen Z-J, Zhong W-X, Yang H, Liu D, Li Y-K. 2022. Anatomical characteristics and significance of the metopism and Wormian bones in dry adult-Chinese skulls. Folia Morphol, https://doi.org/10.5603/FM.a2022.0006
Marathe RR, Yogesh AS, Pandit SV, Joshi M, Trivedi GN. 2010. Inca – interparietal bones in neurocranium of human skulls in central India 2010. JNRP 1(1):14–16, https://doi.org/10.4103/0976-3147.63094
Marti B, Sirinelli D, Maurin L, Carpentier E. 2013. Wormian bones in a general paediatric population. Diagn. Interv. Imaging 94(4):428–32, https://doi.org/10.1016/j.diii.2013.01.001
Masih WF, Gupta S, Chand AE, Jaiswal P, Saraswat PK. 2013. Incidence of Wormian bone in human skulls in Rajasthan. JEMDS 2(9):1001–04, https://doi.org/10.14260/jemds/370
Murlimanju BV, Prabhu LV, Ashraf CM, Kumar CG, Rai R, Maheshwari C. 2011. Morphological and topographical study of Wormian bones in cadaver dry skulls. J Morphol Sci 28(3):176–79.
Murrieta-Angulo S, Tejada-Valdivia CA, Arriola-Guillén LE. 2019. Morphological study of Pterion in skulls of corpses of the Institute of Legal Medicine and Forensic Sciences (Ditanfor), Lima – Peru 2018. Rev Mex Med Forense 4(2):12–23.
Nagarajan K, Ganesh MK. 2017. Variations in the Occurrence of “Os Inca” and its Cranial Deformities in South Indian Dry Skulls. J Pharm Sci and Res 9(2):167–69.
Natsis K, Piagkou M, Lazaridis N, Anastasopoulos N, Nousios G, Piagkos G, Loukas M. 2019. Incidence, number and topography of Wormian bones in Greek adult dry skulls. Folia Morphol 78(2):359–70.
Narayan RK, Kumari S, Verma M. 2019. Prevalence and variety of sutural bones in densely populated East Indian state of Bihar. Ann Acad Med Siles 73:174–81, https://doi.org/10.18794/aams/109153
Nayak SB, Shetty SD. 2019. High Incidence of Sutural Bones, Especially at the Asterion – A South Indian Study. Online J Health Allied Scs 18(8). Available at: https://www.ojhas.org/issue72/2019-4-8.html [Accessed 14 December 2022].
Nikolova SY, Toneva DH, Yordanov YA, Lazarov NE. 2014. Multiple Wormian bones and their relation with definite pathological conditions in a case of an adult cranium. Anthropol Anz 71(3):169–90, https://doi.org/10.1127/0003-5548/2014/0355
Nikolova S, Toneva D, Georgiev I, Yordanov Y, Lazarov N. 2016. Two cases of large bregmatic bone along with a persistent metopic suture from necropoles on the northern Black Sea coast of Bulgaria. Anthropol Sci 124(2):145–53, https://doi.org/10.1537/ase.160530
Nowak J, Pawlak S, Poniewierski W, Adamiec M, Iwańczyk B, Wojtowicz A. 2018. Kości Worma – anatomia czy patologia? Implants. International magazine of oral implantology 13(1):42–45.
O’Loughlin VD. 2004. Effects of Different Kinds of Cranial Deformation on the Incidence of Wormian Bones. Am J Phys Anthropol 123(2):146–55, https://doi.org/10.1002/ajpa.10304
Oguz Ö, Güraslan Şanli S, Bozkir MG, Soames RW. 2004. The pterion in Turkish male skulls. Surg Radiol Anat 26:220–24, https://doi.org/10.1007/s00276-003-0210-2
Pal GP, Routal RV. 1986. A study of sutural bones in different morphological forms of skulls. Anthropol Anz 44(2):169–73.
Panzer S, Peschel O, Haas-Gebhard B, Bachmeier BE, Pusch CM, Nerlich AG. 2014. Reconstructing the Life of an Unknown (ca. 500 Years-Old South American Inca) Mummy – Multidisciplinary Study of a Peruvian Inca Mummy Suggests Severe Chagas Disease and Ritual Homicide. PloS ONE 9(2): e89528, https://doi.org/10.1371/journal.pone.0089528
Patil M, Sheelavant S. 2012. Sexual dimorphism among the Wormian bones in adult human skulls. J Indian Acad Forensic Med 34:124–127.
Praba AMA, Venkatramaniah C. 2015. A study on the occurrence of Wormian bones among the male and female skulls of Tamil Nadu, India. IJAR 3(4):1700–03, http://doi.org/10.16965/ijar.2015.320
Purohit K, Yadav B. 2019. Sutural bones: A study on incidence, laterality and co-relation with cephalic index in dry crania of East Indian ethnicity. Int J Anat Res 7(3.3):6944–51, https://doi.org/10.16965/ijar.2019.265
Raja SK, Siva NRSS. 2016. Incidence of sutural bones at pterion in South Indian dried skulls, Int J Anat Res 4(1):2099–2101, http://doi.org/10.16965/ijar.2016.154
Rajni MG, Shalik RA, Hema N, Renu M, Swati Y. 2018. Incidence of Wormian bones in North India – a study on adult cadavers dried skull. Int J Curr Res 10(8):72372–74, https://doi.org/10.24941/ijcr.31882.08.2018
Ratnaningrum SF. 2020. Identification of sutural bones in Indonesian skulls. Transl Res Anat 18:100061, https://doi.org/10.1016/j.tria.2019.100061
Reicher M, Łasiński W. 2010. Uwagi wstępne (funkcja czaszki). In: Bochenek A, Reicher M, editors. Anatomia człowieka. Tom I. Anatomia ogólna. Kości, stawy i więzadła, mięśnie. Warszawa, Polska: Wydawnictwo Lekarskie PZWL. 299.
Rizvi A, Iwanaga J, Oskouian RJ, Loukas M, Tubbs RS. 2018. Wormian Bone of the Orbit: A Case Report. Cureus 10(8):e3117, https://doi.org/10.7759/cureus.3117
Romero-Reverón R. 2017. Anatomical classification of sutural bones. MOJAP 3(4):130–31, https://doi.org/10.15406/mojap.2017.03.00101
Romero-Reverón R. 2020. False Sutural Bones should have its Own Anatomical Denomination in International Anatomical Terminology? JMA 4(3):134, https://doi.org/10.37421/JMA.2020.4.134
Romero-Reverón R, Arráez-Aybar LA. 2019. Sutural bones: a literature review. Anatomy 13(1):61–65, https://doi.org/10.2399/ana.18.062
Rothman KJ, Greenland S. 2005. Hill’s Criteria for Causality. In: Armitage P and Colton T, editors. Encyclopedia of Biostatistics. 2nd edition. John Wiley & Sons, Ltd. https://doi.org/10.1002/0470011815.b2a03072
Sah SK, Chaudhary D, Pandey N. 2017. Study of metopism and Wormian bones in dry skulls of human cadavers in Nepal. Int J Anat Res 5(1):3443–46, https://doi.org/10.16965/ijar.2016.499
Sanchez-Lara PA, Graham Jr JM, Hing AV, Lee J, Cunningham M. 2007. The Morphogenesis of Wormian Bones: A Study of Craniosynostosis and Purposeful Cranial Deformation. AJMG 143A:3243–51, https://doi.org/10.1002/ajmg.a.32073
Saylisoy S. 2020. Is There a Coexistence of Peritemporal Wormian Bones and Congenital Aural Atresia? JCAT 44:559–61, https://doi.org/10.1097/RCT.0000000000001047
Saxena SK, Chowdhary DS, Jain SP. 1986. Interparietal bones in Nigerian skulls. J Anat 144:235–237.
Semler O, Cheung MS, Glorieux FH, Rauch F. 2010. Wormian Bones in Osteogenesis Imperfecta: Correlation to Clinical Findings and Genotype. AJMG 152(A):1681–87, https://doi.org/10.1002/ajmg.a.33448
Shiv S, Hitesh C, Rajeev K, Ashish T, Bhushan V, Panchal J. 2020. Study of incidence of Wormian bones in southern Haryana – A prospective observational study. IJFCM 7(3):129–33, https://doi.org/10.18231/j.ijfcm.2020.028
Singh R. 2012. Incidence of Sutural Bones at Asterion in Adults Indians Skulls. Int J Morphol 30(3):1182–86.
Smith JD, Genoways HH, Jones Jr JK. 1977. Cranial and dental anomalies in three species of platyrrhine monkeys from Nicaragua. Folia Primatol. 28(1):1–42, https://doi.org/10.1159/000155796
Sreekanth T, Samala N. 2016. Morphological study of Wormian bones in dried adult human skulls in Telangana. IJAR 4(4):3257–62, https://doi.org/10.16965/ijar.2016.454
Standring S. 2016. External skull. In: Black S, editor. Gray’s anatomy: the anatomical basis of clinical practice. USA, Philadelphia: Elsevier. 416–25.
Sudha R, Sridevi C, Ezhilarasi M. 2013. Anatomical variations in the formation of pterion and asterion in South Indian population. IJCRR 5(9):92–101.
Tonni G, Lituania M, Rosignoli L. 2013. Craniosynostosis with Wormian Bone, Bowing of the Long Bones, Unilateral Short Femur, and Focal Fibula Deficiency: A Prenatal Diagnostic Dilemma. JCU 41(7):448–52, https://doi.org/10.1002/jcu.22002
Uchewa OO, Egwu OA, Egwu AJ, Nwajagu GI. 2018. Incidence of Wormian bones in the dried skull of Nigerian males. Int J Anat Var 11(1):32–34, https://doi.org/10.37532/1308-4038.18.11.32
Ukoha U, Oranusi CK, Okafor JI, Udemezue OO, Anyabolu AE, Nwamarachi TC. 2013. Anatomic study of the pterion in Nigerian dry human skulls. Niger J Clin Pract 16(3):325–28, https://doi.org/10.4103/1119-3077.113455
Uday K, Ratna Prabha J. 2016. Wormian bones: Study on dry human skulls in North Karnataka region. Int J Anat Res 4(1):1854–58, http://doi.org/10.16965/ijar.2015.351
Yadav B, Salam R. 2020. A preliminary study on incidence of cranial sutural bones by 3D volume rendering of CT scan in current population in Eastern India. IJAR 8(1.3):7390–94, https://doi.org/10.16965/ijar.2020.110
Veeresh KTM, Kumar V, Yadav N. 2016. The occurrence of Wormian bones within the cranial sutures and their clinical significance. Int J Anat Res 4(4):3082–86, http://doi.org/10.16965/ijar.2016.408
Vishali N, Ebenraj TJ, Rojomon TC. 2012. A rare existence of significant number of wormian bones in the lambdoid suture. IJSR 3(8):671–677.
Walulkar S, Ksheersagar D, Walukar M. 2012. The study of Wormian bones in human skulls in Vidarbha region. PJMS 2(2):18–21.
Zambrano MLA, Kilroy D, Kumar A, Gilchrist MD, Nı Annaidh A. 2021. The presence of Wormian bones increases the fracture resistance of equine cranial bone. PLoS ONE 16(4): e0249451, https://doi.org/10.1371/journal.pone.0249451
Zimmerman H, Yin Z, Zou F, Everett ET. 2019. Interfrontal Bone Among Inbred Strains of Mice and QTL Mapping. Frontiers in Genetics 10:291, https://doi.org/10.3389/fgene.2019.00291
Table 1. Research Characteristics
| Article | Population | Sample | No. of Individuals | Known Sex and Age | Research Method | Comments |
| SCORING = 0 | ||||||
| Ahad and Thenmozhi, 2015 | South Indian | cont? | 25 | A | Asterion bones counted and measured relative to cranial landmarks (bilaterally) | asterion only |
| SCORING = 1 | ||||||
| Saxena et al. 1986 | Nigerian | cont? | 40 | A | Preinterpalietal bones counted | preinterpatietal bones only |
| Singh 2012 | Indian | – | 55 | S, A | Number of WB in asterion noted (bilaterally) | asterion only |
| Masih et al. 2013 | North Indian (Rajasthan) | cont | 150 | S, A | Presence of WB noted | – |
| Durgesh et al. 2015 | Indian (Andhra Pradesh) | cont? | 48 | – | Occurrence of WB noted | WB present only in the lambdoid suture |
| Praba and Venkatramaniah 2015 | Indian (Tamil Nadu) | cont? | 50 | S, A | WB counted in bregma, lambda, pterion and asterion (regardless of the side) | – |
| Raja and Siva 2016 | South Indian | cont? | 75 | A | WB in pterion counted | pterion only |
| Veeresh et al. 2016 | Indian | cont? | 50 | A | Number and location of WB noted (bilaterally); WB photographed | – |
| Sah et al. 2017 | Nepalese | cont? | 80 | A | Presence and localization of WB noted | – |
| Uchewa et al. 2018 | Nigerian | cont | 22 | S, A | WB counted and photographed; lateralization not included | – |
| Rajni et al. 2018 | North Indian | cont/ cont (?) | 55 | A | Number and location of WB noted (bilaterally) | Material including cadaveric and museum samples |
| Ratnaningrum 2020 | Indonesian | cont? | 69 | – | Presence, localization and variations in WB shapes noted (bilaterally) | – |
| SCORING = 2 | ||||||
| Illknur et al. 2009 | Anatolia | cont/arch | 44 | S/–, A | Epipteric bones counted; measurements between pterion and several landmarks taken both in manual and digital way (on photographs) | pterion only; sex (male) assessed on archaeological sample only |
| Murlimanju et al. 2011 | Indian | cont? | 78 | – | Incidence and topographical distribution of WB (bilaterally) | Interparietal and preinterparietal bones not considered as WB |
| Walulkar et al. 2012 | Central Indian (Vidarbha) | cont | 225 | S, A | WB counted bilaterally, measured (max. width x max. length), incidence of various shapes of WB noted | – |
| Sudha et al. 2013 | South Indian | cont | 150 | A | Epipteric and asterion bones noted bilaterally | pterion and asterion only |
| Eboh and Obaroefe 2014 | Nigerian | – | 50 | – | Epipteric bones counted bilaterally; measurements between pterion and several cranial landmarks | pterion only |
| Ghosh et al. 2017 | East Indian | cont | 120 | A | WB counted bilaterally; topographic distribution analysis, symmetry of WB occurrence analysis | – |
| Tallapaneni and Niveditha 2016 | South-Eastern Indian (Telangana) | cont? | 111 | – | Presence of Wormian bones in respect to their location and number noted (bilaterally) | – |
| Uday and Ratna Prabha 2016 | Indian (North Karnataka) | cont | 200 | – | Presence, localization and number of WB noted | – |
| Ukoha et al. 2013 | Nigerian | cont | 56 | A | Frequency of pterion’s types recorded according to Murphy (1956) | pterion only |
| Kalthur et al. 2017 | South Indian (Karnataka) | cont? | 50 | S, A | Epipteric bones counted and measured relative to cranial landmarks (bilaterally) | pterion only |
| Lekshmy et al. 2017 | South Indian | cont | 200 | A | WB counted (bilaterally); topographic distribution of WB | – |
| Nagarajan and Ganesh 2017 | South Indian | cont | 60 | – | Presence and number of Inca bones; skulls with Inca bones photographed | Inca bones only |
| Narayan et al. 2019 | Eastern Indian (Bihar) | cont? | 30 | A | Number of WB and their localization noted bilaterally; cranial index examined | – |
| Nayak and Shetty 2019 | South Indian | – | 27 | S, A | WB noted (regardless of the side), photographs of skulls with WB | – |
| Yadav and Salam 2020 | Eastern Indian | cont | 64 | S, A | WB counted (regardless of the side) | CT scans of head and neck |
| SCORING = 3 | ||||||
| Pal and Routal 1986 | Indian (South Gujarat) | cont | 117 | – | WB noted bilaterally for each morphological type of skull; correlation with cephalic index examined | – |
| Gümüsburun et al. 1997 | Anatolian-Ottoman | arch | 302 | S, A | WB counted bilaterally; examined correlation with the cephalic index (3 morphological forms) | – |
| Govsa et al. 2014 | Turkish | cont | 300 | A | Presence, localization and number of WB noted bilaterally | 3D reconstructions with volume rendering; preinterparietal bones treated separately |
| Oguz et al. 2004 | Turkish | cont? | 26 | S (males only), A | Types of pterions (according to Murphy (1956)) noted; measurements between pterion and selected landmarks; bone thickness measurements | pterion only |
| Cirpan et al. 2014 | West Anatolian | cont | 151 | A | Inca bones counted following the Hauser and De Stefano (1989) and Kadanoff and Mutafov (1968) classification | Inca bones only |
| Cirpan et al. 2016 | West Anatolian | cont? | 150 | A | Presence, frequency and topographic distribution of WB assessed bilaterally; photography | – |
| Durge 2016 | South-Easter Indian (Andhra Pradesh) | cont? | 160 | S, A | WB number and localization noted | – |
| Basnet et al. 2019 | Nepalese | – | 70 | A | WB counted bilaterally; examined correlation with the cephalic index (3 morphological forms) | Inca bones treated separately |
| de Lucena et al. 2019 | North-East Brazilian | cont | 30 | S, A | Numbers of WB in asterion noted; measurements between asterion and several skull landmarks | asterion only |
| Li et al. 2022 | Chinese | cont | 285 | A | Width and length of WB measured, counted (bilaterally); types of WB shapes counted | Inca bones treated separately |
| Murrieta-Angulo et al. 2019 | Peru | cont | 90 | S, A | Type of pterion bilaterally noted according to Murphy’s classification (1956); measurements between the ossification center and zygomatic arch; photographs | pterion only |
| Shiv et al. 2020 | Indian (Southern Haryana) | cont | 130 | S | Location of WB along the coronal, sagittal and lambdoid suture on ectocranial surface noted; photographs | – |
| SCORING = 4 | ||||||
| Finkel 1975 | Lachish | arch | 628 | S | WB presence noted (in lambdoid, sagittal or coronal suture); skull metric differences in relation to WB presence/absence examined | Inca bones excluded |
| Goyal et al. 2019 | North Indian (Haryana) | cont | 147 | – | Presence, number and topographic distribution of WB noted (regardless the side of the skull) | Interparietal and preinterparietal bones not considered as WB |
| Marathe et al. 2010 | Central Indian | cont | 380 | A | Incidence of Inca bone noted; measurements of Inca bone | Inca bones only |
| Natsis et al. 2019 | Greek | cont | 166 | S, A | WB presence noted bilaterally and both exo- and intracranially; WB presence on viscerocranium noted; side asymmetry analysis | Inca bone excluded from the study |
| Purohit and Yadav 2019 | East Indian | – | 180 | S, A | Presence and location of WB noted bilaterally; photographs of WB taken; cephalic index included | Inca bones not counted as WB |
Abbreviations: arch – archaeological sample, cont – contemporary sample; S – sex, A - age. ‘Known age’ was determined positively both when the exact age categories were given or when subjects were assigned the general ‘adult’ age category. ‘Bilaterally’ description in the table refers to the parts of the skull (sutures or landmarks) that appear paired.
Table 2. Wormian Bones Prevalence Among Various Populations Worldwide
| Article | Presence | Total no. | WB in main sutures | WB in specific sites of the skull | |||||||||||||
| C | S | Sq-R | Sq-L | L | B | LL | A-R | A-L | P-R | P-L | I | ||||||
| SCORING = 0 | |||||||||||||||||
| Ahad and Thenmozhi 2015 ⁂ | – | – | – | – | – | – | – | – | – | 12% | 20% | – | – | – | |||
| ∑: 32% | |||||||||||||||||
| SCORING = 1 | |||||||||||||||||
| Saxena et al. 1986 ⁂ | – | – | – | – | – | – | – | – | – | – | – | – | – | II single: 1 (2.5%) PI single: 4 (10%) multi: 1 (2.5%) |
|||
| Singh 2012 ⁂ | – | – | – | – | – | – | – | – | – | ♂: 25.0% ♀: 14.8% |
♂: 10.7% ♀: 14.8% |
– | – | – | |||
| Masih et al. 2013 ⁂ | ♂: 6 (4.1%) ♀: 1 (3.6%) ∑: 7 (4.7%) |
– | – | – | – | – | – | – | – | – | – | – | – | – | |||
| Durgesh et al. 2015 ⁂ | 24.1% | – | – | – | – | – | 14 | – | – | – | – | – | – | – | |||
| Praba and Venkatramaniah 2015 | – | ♂: 56 ♀: 27 |
– | – | – | – | – | ♂: 0 ♀: 0 |
♂: 13 ♀: 1 |
♂: 35 ♀: 19 |
♂: 8 ♀: 7 |
– | |||||
| Raja and Siva 2016 ⁂ | – | – | – | – | – | – | – | – | – | – | – | 1 | 2 | – | |||
| ∑: 3 (4%) | |||||||||||||||||
| Veeresh et al. 2016 ⁂ | 16 (32%) | – | – | 0 | – | – | R: 7 | L: 15 | 0 | 3 (6%) | 1 | 2 | 1 | 0 | – | ||
| ∑: 22 (44%) | ∑: 3 (6%) | ∑: 1 (2%) | |||||||||||||||
| Sah et al. 2017 ⁑⁑ | 55 (68.7%) | – | 0 | 2 (3.6%) | – | – | 35 (63.6%) | 0 | – | 11 (20%) | 7 (12.7%) | – | |||||
| Uchewa et al. 2018 ⁂ | 10 (45.5%) | – | 0 | 0 | – | – | 8 (36.4%) | 0 | – | – | – | ∑: 2 (9.1%) | – | ||||
| Rajni et al. 2018 * | 28 (51%) | – | 0 | 1 (2%) | 2 | 1 | 6 | 8 | 4 (7%) | 1 | 2 | 1 | 0 | – | |||
| ∑: 3 (5%) | ∑: 14 (26%) | ∑: 3 (5%) | ∑: 1 (2%) | ||||||||||||||
| Ratnaningrum 2020 ⁑⁑ | 11 (15.9%) | – | – | – | – | – | 8 (72.7%) | – | – | – | – | 3 (27.3%) | 1 | ||||
| SCORING = 2 | |||||||||||||||||
| Illknur et al. 2009 ⁑ | – | – | – | – | – | – | – | – | – | – | – | Archaeological sample: 1 (6.25%) Contemporary sample: 1 (3.5%) |
– | ||||
| Murlimanju et al. 2011 ⁂ | 57 (73.1%) | – | 1 (1.3%) | 1 (1.3%) | – | – | 44 (56.4%) | 0 | – | ∑: 14 (17.9%) | ∑: 9 (11.%) | – | |||||
| Walulkar et al. 2012 ⁂ | ♂: 63 (39.1%) ♀: 14 (21.9%) ∑: 77 (34.2%) |
– | ♂: 1 (1.3%) ♀: 0 ∑: 1 (1.3%) |
♂: 4 (5.2%) ♀: 0 ∑: 4 (5.2%) |
– | – | ♂: 45 (71.4%) ♀: 12 (85.7%) ∑: 57 (74.2%) |
0 | ♂: 7 (11.1%) ♀: 1 (7.1%) ∑: 8 (10.4%) |
♂: 3 ♀: 1 |
♂: 2 ♀: 2 |
– | – | – | |||
| ∑: 7 (9.1%) | |||||||||||||||||
| Sudha et al. 2013 ⁂ | – | – | – | – | – | – | – | – | – | 13 (8.6%) | 10 (6.6%) | 21 (14%) | 13(8%) | – | |||
| ∑: 23 (7.6%) | ∑: 34 (11.3%) | ||||||||||||||||
| Eboh and Obaroefe 2014 ■ | – | 6 (6%) | – | – | – | – | – | – | – | – | – | 4 (8%) | 2 (4%) | – | |||
| ∑: 6 (6%) | |||||||||||||||||
| Ghosh et al. 2017 | 54 (45%) | 165 | 2.4% | 5.4% | 10.9% | 53.3% | 0.6% | 21.2% | – | – | – | ||||||
| Tallapaneni and Niveditha 2016 ⁂ | 59 (53.1%) | – | 0 | 1 (0.9%) | 3 | 2 | R: 25 | L: 34 | 1 (0.9%) | 9 (8.1%) | 1 | 2 | 2 | 0 | – | ||
| ∑: 5 (4.5%) | ∑: 59 (53.1%) | ∑: 3 (2.7%) | ∑: 2 (1.8%) | ||||||||||||||
| Uday and Ratna Prabha 2016 ⁑⁑ | 113 (56.5%) | – | 0 | 0 | 0 | 5 (4.4%) | R: 21 | L: 43 | 0 | 52 (46%) | 18 | 28 | 1 | 5 | – | ||
| ∑: 64 (56.6%) | ∑: 46 (40.7%) | ∑: 6 (5.3%) | |||||||||||||||
| Ukoha et al. 2013 ⁂ | – | – | – | – | – | – | – | – | – | – | – | 3.6% | 3.6% | – | |||
| Kalthur et al. 2017 * | – | – | – | – | – | – | – | – | – | – | – | R: 24% L: 10% ♂: 18.9% ♀: 11.6% |
– | ||||
| Lekshmy et al. 2017 ⁂ | 123 (61.5%) | – | R: 36 | L: 30 | 34 (27.6%) | 26 | 30 | R: 58 | R: 54 | 3 (2.4%) | 29 (23.6%) | 50 | 48 | 9 | 11 | – | |
| ∑: 66 (53.6%) | ∑: 56 (45.5%) | ∑: 112 (91%) | ∑: 98 (79.7%) | ∑: 20 (16.3%) | |||||||||||||
| Nagarajan and Ganesh 2017 ⁂ | – | – | – | – | – | – | – | – | – | – | – | – | – | single: 7 (87.5%) double:1 (12.5%) ∑: 8 (13.3%) |
|||
| Narayan et al. 2019 ⁑, ⁂ | 13 (43%) | 82 | R: 3 | L: 2 | 0 | 2 | 0 | R: 23 | L: 19 | 0 | 7 (8.2%) | 0 | 0 | 2 | 4 | – – |
|
|
+ 4 bilateral = ∑: 9 (10.6%) |
∑: 2 (2.3%) |
+ 10 bilateral =∑: 52 (61.2%) |
+ 2 bilateral = ∑: 2 (2.3%) |
∑: 6 (7%) | |||||||||||||
| Nayak and Shetty 2019 ⁂ | 24 (88.8%) | – | 2 (7.4%) | – | – | – | 6 (22.2%) | – | 4 (14.8%) | 18 (66.6%) | 2 (7.4%) | – | |||||
| Yadav and Salam 2020 ⁂ | ♂: 11 (30.6%) ♀: 8 (28.6%) ∑: 19 (29.7%) |
– | 1 (5.3%) | 1 (5.3%) | 1 (5.3%) | 15 (78.9%) | – | 4 (21%) | 8 (42.1%) | – | – | – | |||||
| SCORING = 3 | |||||||||||||||||
| Pal and Routal 1986 ⁂ | – | – |
D:0 M: 0 B:0 |
D: 4 (5.8%)
M: 0 B: 0 |
D: 11 (8%) M: 1 (1.3%) B: 1 (5%) |
D: 51 37%) M: 23 (30.3%) B: 9 (45%) |
D: 0 M: 0 B: 0 |
D: 13 (18.8%) M: 4 (10.5%) B: 0 |
D: 8 (5.8%) M: 2 (2.6%) B: 2 (10%) |
D: 8 (5.8%) M: 3 (3.9%) B: 2 (10%) |
– | ||||||
| Gümüsburun et al. 1997 * | – | – | ♂: 4 (2.1%) ♀: 5 (4,5%) ∑: 9 (3%) |
♂: 1 (0.5%) ♀: 4 (3.6%) ∑: 5 (1.6%) |
♂: 14 (7.3%) ♀: 10 (9.1%) ∑: 24 (7.9%) |
♂: 117 (60.9%) ♀: 70 (63.6) ∑: 187 (61.9%) |
♂: 2 (1%) ♀: 0 ∑: 2 (0.7%) |
♂: 26 (13.5%) ♀: 7 (3.6%) ∑: 33 (10.9%) |
♂: 32 (16.7%) ♀: 14 (12.7%) ∑: 46 (15.2%) |
♂: 22 (11.5%) ♀: 8 (7.3%) ∑: 30 (9.9%) |
– | ||||||
| Govsa et al. 2014 ⁂ | 27 | – | – | – | – | – | 12 (4%) | – | – | – | – | – | – |
PI: single:6 multiple: 3 ∑: 9 (3%) II: single: 2 multiple:4 ∑: 6 (2%) |
|||
| Oguz et al. 2004 ⁂ | – | – | – | – | – | – | – | – | – | – | – | ♂: 0 | ♂: 4% | – | |||
| Cirpan et al. 2014 ⁂ | – | – | – | – | – | – | – | – | – | – | – | – | – | 3 (2%) | |||
| Cirpan et al. 2016 ⁑ | 89 (59.3%) | 207 | R:5 (3.3%) | L:5 (3.3%) | 7 (4.7%) | 3 (2%) | 10 (6.7%) | R: 56 (37.3%) | L: 61 (40.7%) | 3 (2%) | 10 (6.7%) | 10 (6.7%) | 11 (7.3%) | 8 (5.3%) | 12 (8%) | – | |
| Durge 2016 | ♂: 30 (41.7%) ♀: 42 (47.7%) ∑: 72 (45%) |
– | ♂: 4 ♀: 0 |
– | – | – | ♂: 22 ♀: 34 |
♂: 2 ♀: 0 |
– | ♂: 2 ♀: 0 |
♂: 0 ♀: 4 |
– | |||||
| Basnet et al. 2019 ⁂ | 62 (88.6%) | – | 3 (4.3%) | 5 (7.1%) | 29 (41.4%) | 43 (61.4%) | 0 | 8 (11.4%) | 17 (24.3%) | 18 (25.7%) | – | ||||||
| de Lucena et al. 2019 ⁂ | – | – | – | – | – | – | – | – | – | ♂: 6.7% ♀: 8.3% |
♂: 11.7% ♀:5% |
– | – | – | |||
| ∑: 19 (31.7%) | |||||||||||||||||
| Li et al. 2022 ⁂ | 182 (63.9%) | – | – | 9 (4.9%) | – | – | 143 (78.6%) | – | 15 (8.3%) | 22 (12.1%) | 63 (34.6%) | 7 (3.8%) | |||||
| Murrieta-Angulo et al. 2019 ⁂■ | – | – | – | – | – | – | – | – | – | – | – | 0 | 0 | – | |||
| Shiv et al. 2020 ⁂ | ♂: 19 (22.1%) ♀: 8 (18.2%) ∑: 27 (20.1%) |
– | 1 (0.8%) | 7 (5.4%) | – | – | 19 (14.6%) | – | – | – | – | – | – | – | |||
| SCORING = 4 | |||||||||||||||||
| Finkel 1975 ⁂ | ♂: 114 ♀: 81 |
– | – | – | – | – | – | – | – | – | – | – | – | – | |||
| Goyal et al. 2019 ⁂ | ♂: 35 (36.1%) ♀: 17 (34%) ∑: 52 (35.3%) |
– | 9 (6.1%) | 7 (4.8%) | – | – | 41 (27.9%) | 0 | 28 (19%) | 3 (2%) | 1 (0.7%) | – | |||||
| Marathe et al. 2010 ⁂ | – | – | – | – | – | – | – | – | – | – | – | – | – | ♂: 3 (1.4%) ♀: 2 (1.2%) ∑: 5 (1.3%) |
|||
| Natsis et al. 2019 ⁂>, ⁑⁑ | ♂: 67 (72.8%) ♀: 57 (77%) ∑: 124 (74.7%) |
– | ♂: 39 (42.4%) ♀: 27 (36.5%) ∑: 66 (39.8%) |
♂: 9 (9.8%) ♀: 9 (12.2%) ∑: 18 (10.8%) |
♂: 7 ♀: 5 ∑: 12 |
♂: 4 ♀: 5 ∑: 9 |
♂: 40 (43.5%) ♀: 34 (45.9%) ∑: 74 (44.6%) |
♂: 2 (2.2%) ♀: – ∑: 2 (1.6%) |
♂: 1 (1.1%) ♀: 2 (2.8%) ∑: 3 (2.4%) |
♂: 15 ♀: 4 ∑: 19 |
♂: 17 ♀: 9 ∑: 26 |
♂: 2 ♀: 4 ∑: 6 |
♂: 2 ♀: 2 ∑: 4 |
– | |||
| Purohit and Yadav 2019 ⁑⁑ | ♂: 90 (72.6%) ♀: 41 (73.2%) ∑: 131 (72.3%) |
– | ♂: 10 (8.9%) ♀: 6 (14.6%) ∑: 14 (10.7%) |
♂: 3 (3.3%) ♀: 5 (12.2%) ∑: 8 (6.1%) |
Parieto-temporal: ♂: 12 (13.2%) ♀: 11 (26.8 %) ∑: 23 (17.6%) Parieto-mastoid: ♂: 7 (7.8%) ♀: 2 (4.9%) ∑: 9 (6.9%) |
♂: 55 (61.1%) ♀: 32 (78%) ∑: 87 (66.4%) |
0 | ♂: 24 (26.7%) ♀: 8 (19.5%) ∑: 32 (24.4%) |
♂: 34 (37.8%) ♀: 15 (36.6%) ∑: (49) 37.4% |
♂: 24 (26.7%) ♀: 13 (31.7%) ∑: 37 (28.2%) |
– | ||||||
* Percentage of WB (total number of bones) in specific sites calculated in relation to whole research sample (divided into sex groups, if distinguished)
⁑ Percentage of WB (total number of bones) in specific sites calculated in relation to group of individuals with present WB only (divided into sex groups, if distinguished)
⁂ Percentage of number of skulls that have WB in specific sites, in relation to whole research sample (divided into sex groups, if distinguished)
⁑⁑ Percentage of number of skulls that have WB in specific sites, in relation to group of individuals with present WB only (divided into sex groups, if distinguished)
■ Both sides of skull treated as an independent record (total number doubled)
Abbreviations: Presence – number and/or percentage of individuals with Wormian bones in sample. Total no. – total sum of Wormian bones in the whole sample. C – coronal suture, S – sagittal suture, Sq-R – right squamous suture, Sq-L – left squamous suture, L – lambdoid suture, B – bregma – LL – lambda, A-R – right asterion, A-L – left asterion, P-R – right pterion, P-L – left pterion, I – Inca bone [II – interparietal, PI – preinterparietal]. Variants of skull morphology: D – dolichocephalic, M – mesocephalic, B – brachycephalic. Parietal notch bones and ossicles in parieto-temporal or parieto-mastoid suture were considered as squamous sutural bones.
Table 3. Wormian Bones Prevalence in Male and Female Skulls (the articles using 3rd computational strategy selected only)
| Article | WB examined in | Males | Females | Statistical significance |
| Finkel 1975 | whole skull | 114 (32.2%) | 81 (29.6%) | not described |
| Marathe et al. 2010 | Inca | 3 (1.428%) | 2 (1.176%) | not described |
| Walulkar et al. 2012 | whole skull | 63 (9.13%) | 14 (21.87%) | not described |
| Singh 2012 | asterion | – (82.14%) | – (85.19%) | not described |
| Masih et al. 2013 | whole skull | – (4.1%) | – (3.6%) | not described |
| Natsis et al. 2019 | whole skull | 67 (72.8%) | 57 (77%) | no significant difference |
| Yadav and Salam 2020 | whole skull | 11 (30.56%) | 8 (28.57%) | not described |
| De Lucena et al. 2019 | asterion | – (18.34%) | – (13.34%) | not described |
| Goyal et al. 2019 | whole skull | 35 (36.08%) | 17 (34%) | no significant difference |
| Shiv et al. 2020 | whole skull | 19 (22.09%) | 8 (18.18%) | not described |
| Yadav and Salam 2020 | whole skull | 11 (30.56%) | 8 (28.57%) | not described |


Received: 17.11.2022; Revised: 12.12.2022; Accepted: 12.12.2022