Available online at: https://doi.org/10.18778/1898-6773.85.4.05
Department of Anthropology, University of Delhi, Delhi
Department of Obstetrics and Gynaecology, Sardar Patel Medical College, Bikaner, Rajasthan, India
Department of Anthropology, University of Delhi, Delhi
Public Health Foundation of India, Gurugram, Haryana, India
Department of Anthropology, University of Delhi, Delhi
ABSTRACT: Low birth weight is one of the leading factors for infant morbidity and mortality. To a large extent affect, various maternal risk factors are associated with pregnancy outcomes by increasing odds of delivering an infant with low birth weight. Despite this association, understanding the maternal risk factors affecting term low birth weight has been a challenging task. To date, limited studies have been conducted in India that exert independent magnitude of these effects on term low birth weight. The aim of this review is to examine the current knowledge of maternal risk factors that contribute to term low birth weight in the Indian population. In order to identify the potentially relevant articles, an extensive literature search was conducted using PubMed, Goggle Scholar and IndMed databases (1993 – Dec 2020). Our results indicate that maternal age, educational status, socio-economic status, ethnicity, parity, pre-pregnancy weight, maternal stature, maternal body mass index, obstetric history, maternal anaemia, gestational weight gain, short pregnancy outcome, hypertension during pregnancy, infection, antepartum haemorrhage, tobacco consumption, maternal occupation, maternal psychological stress, alcohol consumption, antenatal care and mid-upper arm circumference have all independent effects on term low birth weight in the Indian population. Further, we argue that exploration for various other dimensions of maternal factors and underlying pathways can be useful for a better understanding of how it exerts independent association on term low birth weight in the Indian sub-continent.
KEY WORDS: anaemia, gestational weight gain, hypertension, India, Low Birth Weight, maternal age, maternal Body Mass Index, maternal risk factors, obstetric history
The birth weight of the newborn is a prime demographic indicator of the health status of a given society. The decrease and the increase in the mean birth weight of the population are directly linked to the quality of maternity care and living condition of the mothers (Barker 2004). Birth weight also plays a pivotal role in infant and childhood mortality (McCormick 1985).
The birth weight of less than 2500 grams is defined as the Low Birth Weight (LBW), regardless of gestational age (WHO 2004). The LBW can be distinguished into three categories. 1) Premature or Pre-Term LBW (born before 37 completed weeks of gestation or with fewer than 259 days of gestation); 2) Term LBW (born between 37 and 42 completed week of gestation, or between 259 and 293 days of gestation); 3) Post-Term LBW (born after 42 week or 294 days of gestation) (WHO 2004).
LBW can be either caused by a short gestation period or retarded intra-uterine growth, as well as by a combination of both these pathophysiologic conditions (Kramer 1987). Importantly, term LBW and intra-uterine growth restriction do not necessarily reflect the same clinical situation. For example, some new-borns, normally formed and perfectly healthy, are born weighing less than the 10th percentile for their gestational age (Resnik 2002), while others whose birth weight is higher than the 10th percentile may show signs of growth restriction if they come from a uterine environment that thwart the foetus from reaching its full potential for growth (Wollmann 1998).
On average, an infant with LBW has 40 times greater mortality risk than normal weight new-borns (Alexander et al. 2007), while Very Low Birth Weight (<1500 gram), might increase mortality risk up to 100 times (Mayor 2016). LBW infants are also more prone to developing iron deficiency anaemia potentially leading to longer and impaired neurodevelopmental disorders (Long et al. 2012). In addition, several research studies have shown that the impaired growth at birth is linked to an increased risk of developing certain types of chronic disorders at an older age (Sallout et al. 2003), such as diabetes, obesity (Kuhle et al. 2017), endothelial dysfunction (Visentin et al. 2014), non-alcoholic fatty liver diseases (Newton et al. 2017), cardiovascular diseases (Kuhle et al. 2017), asthma (Wjst et al. 1998), hypothermia (Erekia Ebrahim 2015) and chronic kidney disease (Hirano et al. 2016).
Globally, LBW was estimated to comprise 12.4–17.1% of all births (WHO 2019). The prevalence of LBW in Low- and Middle-Income Countries (LMIC) was 91% of the world’s LBW. There were notable global and regional variations in LBW rates. An estimated 14% of neonates exhibited LBW in Sub-Sahara Africa, 12.2% in North Africa, 5.3% in East Asia, 5.4% in Southeast Asia, 9.9% in Western Asia, 8.7% in Latin America and 26.4% in South Asia. Up to one-fourth of all born LBW infants were born in South Asia (Blencowe et al. 2019). In South Asia itself, India heads the list with 18.2% of the infant with LBW as per the National Family Health survey 4 statistics (IIPS and ICF 2017). Compared to prematurity in developed countries, the observed LBW in developing countries, such as India, can be largely attributed to Intra-uterine growth restriction (Saili 2008).
The major problem in the field of public health is to determine factors influencing LBW and to institute therapeutic measures (Velankar 2009). The aetiology of LBW is complex and mainly influences fetal growth, although these factors can be categorised into several different categories on the basis of the locus of their impact: the placenta, the pregnant woman herself, the fetus and finally, factors produced from the interaction of these factors (Institute of Medicine 1985). Although the progress in obstetrical and neonatal care has improved the prognosis for LBW neonates, the best strategy to reduce it is primary prevention by identifying and avoiding the risk factors that led to LBW. This review aims to update the current knowledge and understanding of the maternal risk factors affecting Term LBW in the Indian population.
This comprehensive literature review was conducted using PubMed, Goggle Scholar and IndMed databases from 1993 to December 2021 to identify the relevant articles. The search strategy was developed using combination of medical subject heading (MeSH) terms and words in Title/abstract- (“maternal risk factor” [Title/Abstract] OR “maternal risk factors” [All Fields] OR “risk factor” [All Fields] OR “risk factors” [MeSH Terms]) AND (“infant, low birth weight” [MeSH Terms] OR “infant, low birth weight” [MeSH Terms] OR “low birth weight” [Title/Abstract]). Full text articles that were written in English and relevant to the topic were included in the study. The references of those selected articles were then utilised in a cascade search to obtain more relevant citations.
Inclusion criteria are followed as:
1) Studies published in English 2) Studies related to human 3) Original research articles 4) Natural conception 5) Singleton pregnancy 6) No history of visceral diseases 7) Studies done on Indian population
Exclusion criteria are followed as:
1) Review studies and 2) Systematic review and meta-analysis
Initial search identified 10900 articles for inclusion. After deleting articles not related to humans, 10329 articles were left for consideration. Assessment based on titles and abstracts were carried out to determine the objectives and relevance of the studies, which resulted in exclusion of 9835 studies. The full texts of remaining 494 papers were included for consideration in the study and those articles that neither met the inclusion nor the exclusion criteria were removed from consideration. At this stage of the search process 46 articles were retained while 9 more articles were identified from the references of searched articles and added for final consideration. In total, 55 studies that met all the inclusion and exclusion criteria. The identifying information (such as research objectives, study design, sample size, risk factor, results, and effect size) of those 55 studies are presented in supplementary table 1.
A large number of epidemiological studies in India have shown an increased risk of LBW in extreme reproductive age, i.e., less than 20 years of age (Amin et al. 1993; Fraser et al. 1995; Deshmukh et al. 1998; Agarwal et al. 2005; Joshi et al. 2005; Chen et al. 2007; Dharmalingam et al. 2010; Roy et. al. 2009; Epstein et al. 2013; Raje et al. 2015; Patel et al. 2018; Kumar et al. 2020), and above 30 years of age or both (Cnattingius et al. 1992; Malik et al. 1997; Mondal 2000; Nair et al. 2000; Jha et al. 2009; Ganesh Kumar et al. 2010; Deshpande Jayant et al. 2011; Borah et al. 2016; Patel et al. 2018). The main cause of early conception is a well-established custom of child marriage in India (27% according to NFHS-4 (IIPS and ICF 2017), which is magnified due to the poverty and ignorance (Seth et al. 2018). The devastating effects of early conception also led to an increased risk of stillbirth, abortion, and premature delivery (Rao et al. 2010; Igwegbe et al. 2001). It is generally accepted that women of advanced age (>30 years) exhibit some latent factors that can cause complications in pregnancy, including LBW (Shan et al. 2018; Goisis et al. 2017; Tabcharoen et al. 2009). These latent factors might include an impaired function of the myometrium (Nelson et al. 2013) or a large number of chronic diseases at older ages (Sheen et al. 2018).
Many research studies have demonstrated a direct association between mother’s education level and fetal birth weight. For example, the risk of LBW decreases with an increase in mother’s education level. It might suggest that women with higher levels of education were less prone to neglect health care, have high socio-economic status (SES) (Deshpande Jayant et al. 2011; Mathew et al. 2014), and better decision making regarding health care as well as family planning (Mavalankar et al. 1992; Hirve et al. 1994; Biswas et al. 2008; Subramanyam et al. 2010; Sreeramareddy et al. 2011; Chakraborty et al. 2011; Metgud et al. 2012; Epstein et al. 2013; Kader et al. 2014; Patel et al. 2018; Kumar et al. 2020).
Studies have shown that low SES was associated with high prevalence (11–50%) of LBW (Deshmukh et al. 1998; Nair et al. 2000; Radhakrishnan et al. 2000; Jha et al. 2009; Roy et al. 2009; Chakraborty et al. 2011; Deshpande Jayant et al. 2011; Khattar et al. 2013; Bellad et al. 2012; Kader et al. 2014; Mathew et al. 2014; Kumar et al. 2020). However, the association between SES and LBW should be interpreted in the light of other factors related to SES, such as maternal age, education level, tobacco consumption, gestational weight gain and maternal height (Deshmukh et al. 1998; Roy et al. 2009). In addition, some studies have also reported that low SES can lead to low health consciousness, lower nutritional status and low antenatal attendance, leading to the increased risk of LBW (Nair et al. 2000; Jha et al. 2009; Deshpande Jayant et al. 2011; Mumbare et al. 2012; Chakraborty et al. 2011; Kumar et al. 2020). Moreover, many studies have also found a significant association between SES and birth weight of neonates (Hirve et al. 1994; Deshmukh et al. 1998).
India harbours more genetic diversity compared to other comparable global regions (Majumder 1998). Epidemiological studies have reported large disparities in the prevalence rates of LBW in different racial and ethnic groups (James 1993; Branum et al. 2002) as well as regions (Chakraborty et al. 2011; Epstein et al. 2013). For example, the prevalence of LBW was reported to be the highest in the north India compared to other regions of India (Chakraborty et al. 2011; Epstein et al. 2013). One study reported a significant influence of religion on the prevalence of LBW (i.e. Hindus have more prevalence of LBW compared to Muslims) (Mavalankar et al. 1992). Similarly, the NFHS-4 (National Fertility Health Survey) data also showed that Hindus (18.5%) have more prevalence of LBW than Muslims (17.3%) (IIPS and ICF 2017).
Maternal Parity is defined as the number of pregnancies reaching viable gestational age (>20 weeks), including live birth and still births. The parity is a well-recognised potential indicator for LBW (Shah 2010). LBW has been reported to be significantly high in nulliparous, decrease significantly in multiparous (parity 2–4) and significantly increase in grand multiparous (parity 5–8) (Amin et al. 1993; Mavalankar et al. 1992; Hirve et al. 1994; Malik et al. 1997; Deshmukh et al. 1998; Anand et al. 2000; Nair et al. 2000; Mondal 2000; Chhabra et al. 2004; Joshi et al. 2005; Negi et al. 2006; Roy et al. 2009; Epstein et al. 2013; Patel et al. 2018). The biological mechanisms regarding how parity influences birth weight has not not clearly understood (Shah 2010). It has been hypothesised that the first pregnancy primes the body and led each subsequent pregnancy to be more efficient (Khong et al. 2003). A lower birth weight in nulliparous may be a direct consequence of multiple health factors, such as the overall health, higher rate of smoking before/during pregnancy, low gestational weight gain, higher age, low pre-pregnancy weight, chronic hypertension, and placental vascular disorder (Ego et al. 2008). In contrast, the increase in the incidence of LBW among grand multiparous (Mesleh 1986; Ozumba et al. 1992; Seidman et al. 1991), could be due to chronic hypertension (Al-sibai et al. 1987), loss of elasticity and hyalinisation of blood vessels for uterine rupture (Nelson et al. 2013), uterine atony for post-partum hemorrhage (Israel et al. 1965), atrophy of the endometrium for placenta previa (Evaldson 1990), hyperlordosis, and placenta previa for fetal malpositioning (Tanbo et al. 1987).
Pre-pregnancy weight and BMI are closely linked to pregnancy outcomes. The weight is influenced by both genetic and environmental factors (Kramer 1987). Theoretically, genetic factors determine body weight by controlling adiposity or influencing body mass among infants (O’Rahilly et al. 2006). However, even in the absence of such genetic influences, maternal weight or BMI prior to conception replicates the nutritional reserves that are available for intrauterine growth of the fetus (Kramer 1987). The large meta-analysis of 111,000 births worldwide demonstrated that pre-pregnancy weight has the highest odds ratio for detecting LBW (OR:2.3, 95% CI:2.1–2.5) (Kelly et al. 1996). Young et al. (2015) (Young et al. 2015) showed that one standard deviation increase in pre-pregnancy weight independently associated with 250 grams increase in infant birth weight, which also led to approximately 10% reduction in the risk of delivering LBW infant. Studies conducted in India have shown similar associations in which weight lower than 45 kgs and BMI lower than 20kg/m2 increases the risk for LBW (Hirve et al. 1994; Ganesh Kumar et al. 2010; Singh et al. 2009; Deshpande Jayant et al. 2011).
Maternal stature has been argued to predispose the neonate to LBW and pre-term birth (Chan et al. 2009). Studies have also reported that short maternal stature is associated with LBW (Britto et al. 2013; Inoue et al. 2016). Studies conducted in India have shown similar associations (Deshmukh et al. 1998; Malik et al. 1997; Jha et al. 2009; Sen et al. 2009; Kumar et al. 2010; Mumbare et al. 2012; Deshpande Jayant et al. 2011; Kader et al. 2014; Mathew et al. 2014; Tellapragada et al. 2016; Shivakumar et al. 2018). A WHO collaborative study (1995) (Kelly et al. 1996) showed that a maternal height cut off range of 146–157 cm (OR:1.7, 95% CI:1.6–1.8) is associated with a higher risk for LBW. Higher risk of LBW among shorter mothers can be related to a narrow pelvis, which results in limited space, consequently led to intrauterine growth restriction (IUGR) (Zhang et al. 2007). On the other hand, a study reported a significant association between taller women with cut off ≥170 cm and LBW (Kheirouri et al. 2017). There can be other factors, such as paternal height or other paternal characteristics, that could play an important role in influencing the neonatal size (Veena et al. 2004).
For many decades BMI of mothers has been used as an epidemiological factor predictive of fetal growth (Kramer 1987). A low BMI indicates chronic energy depletion and has been used as an important parameter for assessing nutritional risk in women during the reproductive years (Wynn et al. 1991). It also indicates a wasting of both fat and lean tissue (Allen et al. 1994). Some large epidemiological studies showed that the maternal BMI for gestational age is associated with LBW (Kelly et al. 1996; Brewster et al. 2015). In the same vein, two meta-analyses reported that low BMI among mothers increases the risk of having an LBW infant (Han et al. 2011; Vats et al. 2021) while Indian studies have also shown a similar trend of low maternal BMI (<18.5kg/m2) (Amin et al. 1993; Dharmalingam et al. 2010; Sreeramareddy et al. 2011; Chakraborty et al. 2011; Kader et al. 2014; Raje et al. 2015; Patel et al. 2018). Interestingly, the above-described trend is evenly distributed all over India (i.e., 13 out of 17 states), showing that mothers with low maternal pregnancy BMI are at approximately 30% higher risk of giving birth to a LBW infant with a substantial variation between the states. This variation could be due to the interaction between the proximate factors, such as human development index, antenatal visits, and maternal anaemia (Dharmalingam et al. 2010).
Antecedences of abortion, both induced abortion and miscarriages, have been associated with LBW (Kramer 1987). Some studies have shown that among women with a history of previous miscarriage and induced abortion the risk of LBW is increased more than fourfold (Anand et al. 2000; Negi et al. 2006). However, most of the Indian studies did not differentiate between induced abortion and miscarriages and the biological mechanisms of these two factors regarding their influence on LBW might be different. In induced abortions, for instance, cervical insufficiency from dilation and curettage and uterine adhesions result from a post-abortion complication (Hooker et al. 2016). On the other hand, the cervical incompetence has been found to be a major predictor of miscarriages, along with genetic, immunological and uterine abnormalities (Jeve et al. 2014).
A history of LBW in previous pregnancy increases the risk of LBW in the current pregnancy, and this risk continues even after controlling for the socio-demographic and obstetric factors (Anand et al. 2000; Idris et al. 2000; Negi et al. 2006; Roy et al. 2009; Singh et al. 2009; Deshpande Jayant et al. 2011; Metgud et al. 2012; Khattar et al. 2013).
Anaemia during pregnancy is a major public health concern that affects almost two-third of pregnant women in developing countries and contributes to maternal morbidity and LBW infant (Figueiredo et al. 2018). A large cohort study from China showed that the risk of anaemia increases more than twofold from 13th week to 32nd week of pregnancy (Zhang et al. 2009). According to NFHS-4 statistics (IIPS and ICF 2017), the prevalence of anaemia was estimated to be 50.3% in India. Anaemia during pregnancy is a well-known and established physiological fact. The haemoglobin (Hb) and haematocrit concentration typically decreases during the first 13 weeks and reach the lowest level at the end of 28th week of pregnancy, and increases again during the third trimester (Laflamme 2010). The physiological drop in Hb and haematocrit concentration is attributed to an increase in plasma volume which, in turn, results in a decrease in blood viscosity (Carlin et al. 2008) leading to a better circulation in the placenta (Tan et al. 2013). When the Hb concentration levels were reduced to <11g/dL, changes in placental angiogenesis were observed, limiting the availability of oxygen to the fetus and consequently causing potential restriction of intrauterine growth and LBW (Stangret et al. 2017). Studies conducted in India have suggested a similar pattern showing that maternal Hb levels below 11g/dL were at increased risk of having LBW compared to healthy pregnant women (Mavalankar et al. 1992; Deshmukh et al. 1998; Idris et al. 2000; Anand et al. 2000; Dharmalingam et al. 2010; Ganesh Kumar et al. 2010; Deshpande Jayant et al. 2011; Khattar et al. 2013; Borah et al. 2016; Ahankari et al. 2017; Patel et al. 2018; Shankar et al. 2019).
The weight of women increases during the pregnancy which, in turn, affects the inter-uterine growth (Hector et al. 2013). Weight gain during pregnancy is divided into four components 1) Increase plasma volume 2) growth of breast and uterine tissues 3) laying down the fat stores, and 4) growth of the placenta, amniotic fluid and fetus (Kramer 1987). The first three components serve as an energy source to the growing fetus, and a decline in those will result in a decrease in the overall birth weight (Kramer 1987). Thus, weight gain is a factor that affects the size of the fetus (Hector et al. 2013). In 2009, the Institute of Medicine (IOM), USA, published (Rasmussen and Yaktine 2009) the revised Gestational weight gain (GWG) guidelines that are based on pre-pregnancy ranges for underweight, normal weight, overweight and obese women to gain 12.5–18 kg, 11.5–16 kg, 7–11.5 kg, and 5–9 kg respectively. Although these recommendations have been widely accepted (Davies et al. n.d.), they were based on parameters of American women (Kelly et al. 1996), and Asian women parameters, whose BMI classification differs for the one used in the west (WHO 2000), were not considered. Therefore, the applicability of such guidelines to Asian countries is debated [81]. In addition, there is also no such GWG recommendation available for Asian women. As there are not enough publications based on use of IOM guidelines among the Indians and other Asian women (Arora et al. 2019). However, in the absence of India specific GWG guidelines, it was observed that the weight gain of less than 5 kg increases more than six-fold chance of being LBW (Roy et al. 2009; Metgud et al. 2012; Hanumant Dandekar et al. 2014).
Birth spacing contributes to adverse birth outcomes (Kramer 1987). The short interval between the pregnancies increased the risk of LBW and other obstetric complications (Gibbs et al. 2012; Kozuki et al. 2013; Allis 1983). Indian studies have found a similar significant association between pregnancy interval of less than 24 months and obstetrical complication (Deshpande Jayant et al. 2011; Metgud et al. 2012). While other studies have mentioned that the increased risk of LBW when the pregnancy interval is shorter than 12 months (Negi et al. 2006; Roy et al. 2009) or 18 months (Borah et al. 2016). The biological mechanism behind this is not yet clearly understood, but it is likely that pregnancies that occur before the restoration of energy balance, maternal hormones and repletion of maternal resources can lead to health complications in subsequent pregnancies (Conde-Agudelo et al. 2012).
Hypertension during pregnancy is classified as gestational hypertension, pre-eclampsia, severe pre-eclampsia, or eclampsia (Mammaro et al. 2009). Gestational hypertension is diagnosed when the blood pressure equals to or is greater than 140/90mmHg without proteinuria after 20 weeks of gestation (Mammaro et al. 2009). Hypertension associated with the symptoms of proteinuria, seizure or both can indicate the presence of pre-eclampsia and eclampsia (Visintin et al. 2010). As per the prevailing hypothesis of the “ischemic model”, hypertension decreases uteroplacental perfusion by reducing placental blood flow (van Beek et al. 1997) which, in turn, results in the decreased fetal growth with an increased risk of pre-term birth and LBW (Misra 1996). Studies in India also showed a significant association between increased risks of LBW with pregnancy induced hypertension (Metgud et al. 2012; Deshpande Jayant et al. 2011) or Pre-eclampsia and Eclampsia (Idris et al. 2000; Singh et al. 2009).
Bacterial, viral and parasite infections experienced during pregnancy can affect placental development and function, which can lead to IUGR (Adams Waldorf et al. 2013). Infections, such as Treponema pallidum (syphilis) (De Santis et al. 2012), HIV (Xiao et al. 2015), Plasmodium falciparum or Plasmodium vivax (Rijken et al. 2012), Trypanosoma cruzi (Chagas’) (Cevallos et al. 2014) have been shown to be associated with LBW. In contrast, only two Indian studies that have covered a very broad spectrum for infections have been reported (Tellapragada et al. 2016; Idris et al. 2000) and there has been a paucity of studies related to infections during pregnancy in India to support the above-mentioned associations with various pathogens.
Other widely reported infection in pregnancy, also in India, has been a periodontal infection that also has been regarded as a potential risk factor for LBW (Offenbacher et al. 1996) (Deshpande Jayant et al. 2011; Mathew et al. 2014; Basha et al. 2015; Tellapragada et al. 2016).
Antepartum haemorrhage (APH) is a bleeding from or into the genital tract, usually occurring from 24th week of pregnancy onwards and prior to the birth of the fetus (WHO 2011). It is an important predictor of pregnancy outcomes (Bener et al. 2012). It has been estimated that 70% of women who bleed in the last half of the pregnancy have an equal chance of exhibiting either placenta previa , or abruption placentae while in the remaining 30% five out of six cases is unexplained due to indeterminate site of bleeding and one out of six cases is caused by extra placental factors (Konar 2014). Several worldwide studies showed an increased risk of LBW for bleeding in the late pregnancy (Bener et al. 2012) and studies in India support (OR:3.2, P<0.01) the above-mentioned observation (Idris et al. 2000).
Tobacco smoking by women of childbearing age has long been suggested to be one of the most critical factors associated with maternal-fetal health. Tobacco consumption affects the intrauterine environment through several mechanisms (Scholl et al. 1986; Kramer 1987) of which the most commonly reported involve mediators, such as carbon monoxide and nicotine. Carbon monoxide decreases the oxygen-carrying capacity and increases carboxy-hemoglobin, which leads to less release of oxygen to the fetal tissues (Longo 1977). Nicotine, on the other hand, works as an appetite suppressant and results in a rapid increase of catecholamines consequent to uterine vasoconstriction (Quigley et al. 1979). Further, the cyanide compound present in smoke leads to mediated inferences with fetal oxidative metabolism (Andrews 1973). Large epidemiological studies have shown a significant association between tobacco consumption and LBW, even after controlling for confounding factors (Coutinho et al. 2009; Dietz et al. 2010). Similar findings have also been also reported from India (Deshmukh et al. 1998; Deshpande Jayant et al. 2011).
Active smoking directly affects maternal-child health, but studies show that passive smoking also affects maternal-child health through inhaled air pathways (Ward et al. 2007; Pogodina et al. 2009). Compered to men, females are more exposed to the ill effects of tobacco smoke due to passive smoking in their homes or outside as environmental conditions, such as overcrowding and poor ventilation at home (Khattar et al. 2013). Studies conducted in developed countries have shown an association between maternal environment tobacco smoke (ETS) exposure and LBW with increased odds from 1.0 to 2.2 (Ward et al. 2007; Pogodina et al. 2009). Most of the Indian studies also shown a stronger association between ETS exposure and LBW neonate (Gupta et al. 2004; Khattar et al. 2013; Metgud et al. 2012).
Many studies confirm a significant association between maternal occupation and LBW worldwide (Meyer et al. 2008; Casas et al. 2015). One study showed that a moderate to vigorous activity throughout pregnancy may enhance birth weight while severe activity may lead to lighter offspring (Pivarnik 1998). Choudhary et al. (2013) showed that in India daily calorie, mother occupation, and the daytime rest taken were inter-related and significantly associated with LBW. This study also reported that daily calorie was less than 2000 kcal, daytime rest of less than 1 hour and worked as labourer increases the risk of LBW (Choudhary et al. 2013). Moreover, several studies have also shown that mothers who were unemployed, farm labourers during pregnancy have a higher risk of LBW compared to employed (professional/clerical services et al. 2011; Epstein et al. 2013; Kumar et al. 2020).
Maternal psychological stress factors include stressful life changing events, anxiety, mental illness, abuse, and unwanted pregnancy. These factors have been shown to be associated with LBW, prematurity and IUGR (Rondó et al. 2003; Chhabra 2007; Sarkar 2008). The reason behind this may be related to the release of catecholamines or corticosteroids, which increases the vulnerability to infectious diseases (like chorioamnionitis) due to a higher degree of neuromuscular reactivity and the secretion of oxytocin. These factors might induce the placental hypotension and consequent restriction of oxygen and nutrient to the fetus, leading to growth impairment or precipitation of pre-term delivery (Omer 1986; Copper et al. 1996). Stressed women have been reported to more often smoke cigarettes or use a substance such as alcohol and caffeine (McAnarney et al. 1990). Rondo et al. (2003), in their study, observed that maternal distress was associated with LBW and prematurity and also reported an interaction between distress and smoking (Rondó et al. 2003). In India, only one study showed an association between local crime involving a harassment of women and girls with LBW (Baker et al. 2018), although no other major study has investigated this association.
A systematic review and meta-analysis revealed that, compared to abstainers (i.e., those who consume less than 19-gram pure alcohol per day) a heavy alcohol consumption during pregnancy increases the risk of LBW, pre-term birth and small for gestational age, whereas light to moderate alcohol consumption showed no effect (although no data from Asia was included in this review (Patra et al. 2011). Another study from Asia revealed that maternal alcohol consumption of more than 1 gram per day during pregnancy was significantly associated with a risk of pre-term birth, but not with LBW and small for gestational age (Miyake et al. 2014). However, the results of this study might not be representative of all Asian countries as the study did not cover the whole of Asia. In India, no study reported the association between alcohol consumption and LBW. One reason can be its low prevalence of 5.8% in India as reported by the Gender, Alcohol, and Culture: An International Study (GENACIS) project (WHO 2005).
Studies in developing countries have provided evidence that improvement in Antenatal Care (ANC) can significantly reduce the incidence of LBW (Mahumud et al. 2017; Zhou et al. 2019). Quality of ANC, as recommended by WHO, includes at least four standard qualities ANC visits, comprising interventions, such as tetanus toxoid vaccination, screening as well as treatment for infections and identification of warning signs during pregnancy (WHO 2016). These recommendations vary worldwide; in India, for instance, an adequate ANC was considered when the pregnant women were registered at any time during pregnancy, had at least three ANC check-ups, was adequately vaccinated against tetanus, had consumed at least 100 tablets of iron and folic acid, was not involved in hard work and had taken adequate rest during pregnancy (minimum 2 hours sleep during day and 8 hours sleep during the night) (Mumbare et al. 2012). Several studies in India have established a significant relationship between these factors and LBW (Deshpande Jayant et al. 2011; Metgud et al. 2012; Epstein et al. 2013; Choudhary et al. 2013; Negandhi et al. 2014; Mumbare et al. 2012). Other studies have reported a more significant link when registration with ANC was late (Negi et al. 2006; Singh et al. 2009) or a number of ANC visits was lower (Malik et al. 1997; Idris et al. 2000; Agarwal et al. 2005; Dharmalingam et al. 2010; Jha et al. 2009; Khattar et al. 2013; Kader et al. 2014; Mathew et al. 2014). Another study showed a lower incidence of LBW among mothers who received average quality ANC (18.5%), and good quality ANC (13.5%) (OR=1.45, 95% CI: 1.13–1.87, p <0.05) (Nair et al. 2000). To summarise the above studies, adequate ANC care prevents LBW, regardless of the presence of possible confounding factors.
Mid-upper arm circumference (MUAC) is a good indicator for identifying chronic energy deficiency in the body (James et al. 1994) and plays an important role in the determinant of LBW. A WHO collaborative study (1995) (Kelly et al. 1996) showed that MUAC cut-off values of <21–23 cm (OR: 1.9, 95% CI: 1.7–2.1) were at higher risk for LBW. In another study, Mohanty et al. (2006) reported MUAC of 395 pregnant women in the first trimester and found that MUAC ≤22.5 cm was the best cut off value to predict LBW (Mohanty et al. 2006). Several other studies conducted in different parts of India showed a significant association between the birth weight of neonates and MUAC. According to those studies, MUAC was the best surrogate measure for LBW (Sen et al. 2009; Shrivastava et al. 2016) as MUAC was insensitive to the changes experienced during the pregnancy (Katz et al. 2010).
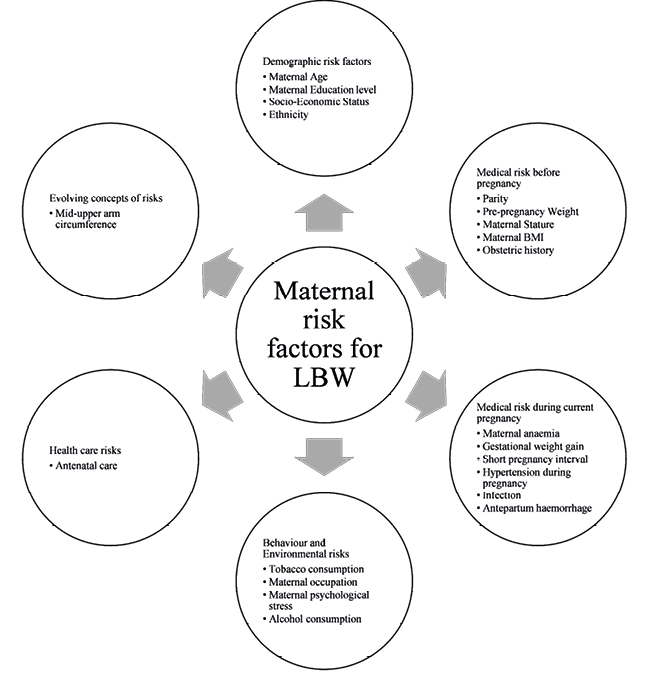
Low birth weight has been known to cause numerous adverse effects among neonates and infants. This literature review suggests that maternal age, educational status, socio-economic status, ethnicity, parity, pre-pregnancy weight, maternal stature, maternal body mass index, obstetric history, maternal anaemia, gestational weight gain, short pregnancy outcome, hypertension during pregnancy, infection, antepartum haemorrhage, tobacco consumption, maternal occupation, maternal psychological stress, alcohol consumption, antenatal care and mid-upper arm circumference are independently associated with term LBW in the Indian population. The awareness about the various aspect of maternal risk factors during pregnancy and understanding general pathways underlying Term LBW can be potentially very beneficial for the healthcare providers to apply the preventive measures and the necessary interventions. The prenatal screening should be started so the high-risk pregnant women will be clearly marked and given a plan for pregnancy with regular advice. A suggested summary for maternal risk factors for low birth weight is depicted in figure 1.

Fig. 1. Summary of Maternal risk factors for LBW in Indian population
Conflict of interest
The authors report no conflict of interest.
Ethical approval
The manuscript is a narrative review paper and did not require any clinical trials registration.
Authors’ contributions
The topic was conceived by HV, RS and VG. The literature review was performed by HV and VG who also constructed the project outline and drafted the manuscript. The figure was developed by HV. The critical review of manuscript was done by MPS, GKW, VG.
Funding
This study has been supported by Wellcome/DBT India Alliance,Hyderabad, India, Intermediate fellowship award to Vipin Gupta(grant reference: IA/CPHI/16/1/502623) and Institute of Eminence, Delhi University, India.
*Corresponding author: Vipin Gupta, Department of Anthropology, University of Delhi, India; e-mail: drvipiing@gmail.com
Adams Waldorf KM, McAdams RM. 2013. Influence of infection during pregnancy on fetal development. Reproduction 146(5):151.
Agarwal N, Reddaiah VP. 2005. Factors affecting birth weight in a sub-urban community: A study in a secondary level hospital in Delhi. Health and Population: Perspectives and Issues 28(4):189–96.
Ahankari AS, Myles PR, Dixit J V, Tata LJ, & Fogarty AW. 2017. Risk factors for maternal anaemia and low birth weight in pregnant women living in rural India: a prospective cohort study. Pub Health 151:63–73.
Al-sibai MH, Rahman MS, Rahman J. 1987. Obstetric problems in the grand multipara: a clinical study of 1330 cases. J Obstet Gynaecol 8(2)135–8.
Alexander GR, Wingate MS, Mor J, Boulet S. 2007. Birth outcomes of Asian-Indian-Americans. Int J Obstet Gynaecol 97(3):215–20.
Allen LH, Lung’aho MS, Shaheen A, Harrison GG, Neumann C, Kirksey A. 1994. Maternal body mass index and pregnancy outcome in the Nutrition Collaborative Research Support Program. Eur J Clin Nutr 48S:68–76.
Allis PA. 1983. Low birth weight deliveries-management of the subsequent pregnancy. J Obstet Gynaecol 3(3):161–62.
Amin N, Abel R, Sampathkumar V. 1993. Maternal risk factors associated with low birth weight. Indian J Pediatr 60(2):269–74.
Anand K, Garg BS. 2000. A Study of Factors Affecting LBW. Indian J Community Med 25(2):57–61.
Andrews J. 1973. Thiocyanate and smoking in pregnancy. BJOG: An Int J Obstet Gynaecol 80(9):810–14.
International Institute for Population Sciences (IIPS) and ICF. 2017. National Family Health Survey (NFHS-4), 2015–16: India. Mumbai: IIPS.
UNICEF-WHO. 2019. UNICEF-WHO Low birthweight estimates: Levels and trends 2000–2015 | UNICEF.
Arora P, Tamber Aeri B. 2019. Gestational Weight Gain among Healthy Pregnant Women from Asia in Comparison with Institute of Medicine (IOM) Guidelines-2009: A Systematic Review. J Pregnan 2019.
Baker KK, Story WT, Walser-Kuntz E, Zimmerman MB. 2018. Impact of social capital, harassment of women and girls, and water and sanitation access on premature birth and low infant birth weight in India. PLoS ONE 13(10):1–18.
Barker D. 2004. Developmental origins of adult health and disease. J Epidemiol Community Health 58(2):114–5.
Basha S, Swamy HS, Mohamed RN. 2015. Maternal periodontitis as a possible risk factor for preterm birth and low birth weight – A prospective study. Oral Health Prev Dent 13(6):537–44.
World Haalth Organisation. 2000. The Asia-Pacific perspective : redefining obesity and its treatment.
van Beek E, Peeters LL. 1997. The pathogenesis of preeclampsia. Nederlands tijdschrift voor geneeskunde 141(28):1379–84.
Bellad M et al. 2012. Consanguinity, prematurity, birth weight and pregnancy loss: a prospective cohort study at four primary health center areas of Karnataka, India. J Perinatol 32:431–7.
Bener A, Salameh KMK, Yousafzai MT, Saleh NM. 2012. Pattern of Maternal Complications and Low Birth Weight: Associated Risk Factors among Highly Endogamous Women. ISRN Obstet Gynecol 2012:540495.
Biswas R, Dasgupta A, Sinha RN, Chaudhuri RN. 2008. An epidemiological study of low birth weight newborns in the district of Puruliya, West Bengal. Indian J. Public Health 52(2):65–71.
Blencowe H, Krasevec J, de Onis M, Black RE, Xiaoyi A, Stevens GA et al. 2019. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. The Lancet Global Health 7(7):e849–e60.
Borah M, Agarwalla R. 2016. Maternal and socio-demographic determinants of low birth weight (LBW): A community-based study in a rural block of Assam. J Postgrad Med 62(3):178–81.
Branum AM, Schoendorf KC. 2002. Changing patterns of low birthweight and preterm birth in the United States, 1981–98. Paediatr Perinat Epidemiol 16(1):8–15.
Brewster AJ, Hardock V, Bhattacharya S. 2015. Exploring the relationship between maternal body mass index and offspring birth weight: Analysis of routinely collected data from 1967 to 2010 in Aberdeen, Scotland. J Obstet Gynaecol 35(8):810–6.
Britto RPDA et al. 2013. Influence of maternal height and weight on low birth weight: A cross-sectional study in poor communities of northeastern Brazil. PLoS ONE 8(11): e80159.
Carlin A, Alfirevic Z. 2008. Physiological changes of pregnancy and monitoring. Best Pract Res Clin Obstet Gynaecol 22(5):801–23.
Casas M, Cordier S, Martinez D, Barros H, Bonde JP, Burdorf A, et al. 2015. Maternal occupation during pregnancy, birth weight, and length of gestation: combined analysis of 13 European birth cohorts. Scand J Work Environ Health 41(4):384–96.
Cevallos AM, Hernández R. 2014. Chagas’ disease: Pregnancy and congenital transmission. Biomed Res Int 2014:401864.
Chakraborty P, Anderson AK. 2011. Maternal autonomy and low birth weight in India. J Womens Health 20(9):1373–82.
Chan BCP, & Lao TTH. 2009. The impact of maternal height on intrapartum operative delivery: A reappraisal. J Obstet Gynaecol Res 35(2):307–14.
Chen X-K, Wen SW, Fleming N, Demissie K, Rhoads GG, Walker M. 2007. Teenage pregnancy and adverse birth outcomes: a large population based retrospective cohort study. Int J Epidemiol 36(2):368–73.
Chhabra P, Sharma AK, Grover VL, Aggarwal OP. 2004. Prevalence of low birth weight and its determinants in an urban resettlement area of Delhi. Asia Pac J Public Health 16(2):95–8.
Chhabra S. 2007. Physical violence during pregnancy. J Obstet Gynaecol 27(5):460–3.
Choudhary AK, Choudhary A, Tiwari SC, Dwivedi R. 2013. Factors associated with low birth weight among newborns in an urban slum community in Bhopal. Indian J Public Health 57(1):20–3.
Cnattingius S, Forman MR, Berendes HW, Isotalo L. 1992. Delayed Childbearing and Risk of Adverse Perinatal Outcome: A Population-Based Study. JAMA 268(7):886–90.
Conde-Agudelo A, Rosas-Bermudez A, Castaño F, Norton MH. 2012. Effects of Birth Spacing on Maternal, Perinatal, Infant, and Child Health: A Systematic Review of Causal Mechanisms. Stud Fam Plann 43(2):93–114.
Copper RL, Goldenberg RL, Das A, Elder N, Swain M, Norman G, et al. 1996. The preterm prediction study: maternal stress is associated with spontaneous preterm birth at less than thirty-five weeks’ gestation. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol 175(5):1286–92.
Coutinho PR, Cecatti JG, Surita FG., de Souza JP, de Morais SS. 2009. Fatores associados a baixo peso ao nascer em uma série histórica de partos em Campinas, Brazil. Revista da Associacao Medica Brasileira 55(6):692–9.
Davies G, Maxwell C, McLeod L, Gagnon R, Basso M, Bos H, et al. 2010. SOGC Clinical Practice Guidelines: Obesity in pregnancy. No. 239, February 2010. Int J Gynaecol Obstet 110(2):167–73.
Deshmukh JS, Motghare DD, Zodpey SP, Wadhva SK. 1998. Low birth weight and associated maternal factors in an urban area. Indian Pediatrics 35(1):33–6.
Deshpande Jayant D, Phalke DB, Bangal VB, D Peeyuusha BS. 2011. Maternal Risk Factors for Low Birth Weight Neonates : a Hospital Based Case-Control Study in Rural Area of Western Maharashtra. Indian J Community Med 2(3):394–8.
Dharmalingam A, Navaneetham K, Krishnakumar CS. 2010. Nutritional status of mothers and low birth weight in India. Matern Child Health J 14(2):290–8.
Dietz PM, England LJ, Shapiro-Mendoza CK, Tong VT, Farr SL, Callagham WM. 2010. Infant morbidity and mortality attributable to prenatal smoking in the U.S. Am J Prev Med 39(1):45–52.
Ego A, Subtil D, Grange G, Thiebaugeorges O, Senat M-V, Vayssiere C, Zeitlin J. 2008. Should parity be included in customised fetal weight standards for identifying small-for-gestational-age babies? Results from a French multicentre study. BJOG 115(10):1256–64.
Epstein MB, Bates MN, Arora NK, Balakrishnan K, Jack DW, Smith KR. 2013. Household fuels, low birth weight, and neonatal death in India: The separate impacts of biomass, kerosene, and coal. Int J Hyg Environ Health 216(5):523–32.
Erekia Ebrahim TS. 2015. Proportion of Neonatal Hypothermia and Associated Factors among New-borns at Gondar University Teaching and Refferal Hospital, Northwest Ethiopia: A Hospital Based Cross Sectional Study. General Medicine 3(04):1–7.
Evaldson GR. 1990. The grand multipara in modern obstetrics. Gynecol Obstet Investig 30(4):17–23.
Figueiredo ACMG, Gomes-Filho IS, Silva RB, Pereira PSP, Da Mata FAF, Lyrio AO, Souza ES et al. 2018. Maternal anemia and low birth weight: A systematic review and meta-analysis. Nutrients 10(5):601.
Fraser AM, Brockert JE, Ward RH. 1995. Association of Young Maternal Age with Adverse Reproductive Outcomes. N Engl J Med 332(17):1113–8.
Ganesh Kumar S, Harsha Kumar HN, Jayaram S, Kotian MS. 2010. Determinants of low birth weight: A case control study in a district hospital in Karnataka. Indian J Pediatr 77(1):87–9.
Gibbs CM, Wendt A, Peters S, Hogue CJ. 2012. The impact of early age at first childbirth on maternal and infant health. Paediatr Perinat Epidemiol 26S:259–84.
Goisis A, Remes H, Barclay K, Martikainen P, Myrskylä M. 2017. Advanced Maternal Age and the Risk of Low Birth Weight and Preterm Delivery: a Within-Family Analysis Using Finnish Population Registers. Am j Epidemiol 186(11):1219–26.
Gupta PC, Sreevidya S. 2004. Smokeless tobacco use, birth weight, and gestational age: Population based, prospective cohort study of 1217 women in Mumbai, India. BMJ 328(7455):1538–40.
Han Z, Mulla S, Beyene J, Liao G, McDonald SD. 2011. Maternal underweight and the risk of preterm birth and low birth weight: A systematic review and meta-analyses. Int J Epidemiol 40(1):65–101.
Hanumant Dandekar R, Shafee M, Sinha SP. 2014. Prevalence and risk factors affecting low birth weight in a district hospital at Perambalur, Tamilnadu. Int J Community Med Public Health 3(2).
Hector D, Hebden L. 2013. Prevention of excessive gestational weight gain: An evidence review to inform policy and practice.
Hirano D, Ishikura K, Uemura O, Ito S, Wada N, Hattori M, Ohashi Y, Hamasaki Y, Tanaka R, Nakanishi K, Kaneko T, Honda M; Pediatric CKD Study Group in Japan in conjunction with the Committee of Measures for Pediatric CKD of the Japanese Society of Pediatric Nephrology. 2016. Association between low birth weight and childhood-onset chronic kidney disease in Japan: a combined analysis of a nationwide survey for paediatric chronic kidney disease and the National Vital Statistics Report. Nephrol Dial Transplant 31(11):1895–1900.
Hirve SS, Ganatra BR. 1994. Determinants of low birth weight: a community based prospective cohort study. Indian pediatr 31(10):1221–1225.
Hooker A, Fraenk D, Brölmann H, Huirne J. 2016. Prevalence of intrauterine adhesions after termination of pregnancy: a systematic review. Eur J Contracept Reprod Health Care 21(4):329–335.
Idris MZ, Gupta A, Mohan U, Srivastava AK, Das V. 2000. Maternal Health and Low Birth Weight Among Institutional Deliveries. Indian J Community Med XXV(4):156–160.
Igwegbe AO, Udigwe GO. 2001. Teenage pregnancy: Still an obstetric risk. J Obstet Gynaecol 21(5):478–481.
Inoue S, Naruse H, Yorifuji T, Kato T, Murakoshi T, Doi H, Subramanian SV. 2016. Association between Short Maternal Height and Low Birth Weight: a Hospital-based Study in Japan. J Korean Med Sci. 31(3):353–9.
Institute of Medicine. 1985. Preventing Low Birthweight. Washington, DC: The National Academies Press.
James SA. 1993. Racial and ethnic differences in infant mortality and low birth weight. A psychosocial critique. Ann Epidemiol 3(2):130–136.
James WP, Mascie-Taylor GC, Norgan NG, Bistrian BR, Shetty PS, Ferro-Luzzi A. 1994. The value of arm circumference measurements in assessing chronic energy deficiency in Third World adults. Eur J Clin Nutr 48(12):883–94.
Jeve YB, Davies W. 2014. Evidence-based management of recurrent miscarriages. J Hum Reprod Sci 7(3):159–169.
Jha SK, Misra CP, Hussain MA. 2009. Determinants of low birth weight: findings from a community based study in a rural area of Varanasi. Indian J Community Health 21(1):18–22.
Joshi HS, Subba SH, Dabral1 SB, Dwivedi S, Kumar D, Singh S. 2005. Risk Factors Associated with Low Birth Weight in Newborns. Indian J Community Med 30(4):142–143.
Kader M. Perera NKP. 2014. Socio-economic and nutritional determinants of low birth weight in India. N Am J Med Sci 6(7):302–308.
Katz J, Khatry SK, LeClerq SC, West KP, Christian P. 2010. The post-partum mid-upper arm circumference of adolescents is reduced by pregnancy in rural Nepal. Matern Child Nutr 6(3):287–295.
Kelly A, Kevany J, De Onis M, Shah PM. 1996. A WHO Collaborative Study of Maternal Anthropometry and Pregnancy Outcomes. Int J Gynaecol Obstet 53(3):219–233.
Khattar D, Awasthi S, Das V. 2013. Residential environmental tobacco smoke exposure during pregnancy and low birth weight of neonates: Case control study in a public hospital in Lucknow, India. Indian Pediatr 50(1):134–138.
Kheirouri S, Alizadeh M. 2017. Impact of prenatal maternal factors and birth order on the anthropometric status of newborns in Iran. J Biosoc Sci 49(2):251–264.
Khong TY, Adema ED, Erwich JJHM. 2003. On an anatomical basis for the increase in birth weight in second and subsequent born children. Placenta 24(4):348–353.
Konar H. 2014. DC Dutta’s Textbook of Obstetrics. Jaypee Brothers Medical Publishers Pvt. Limited.
Kozuki N, Lee AC, Silveira MF, Victora CG, Adair L, Humphrey J, Ntozini R, Black RE, Katz J; Child Health Epidemiology Reference Group Small-for-Gestational-Age-Preterm Birth Working Group. The associations of birth intervals with small-for-gestational-age, preterm, and neonatal and infant mortality: a meta-analysis. BMC Public Health. 2013;13 Suppl 3(Suppl 3):S3.
Kramer MS. 1987. Determinants of low birth weight: Methodological assessment and meta-analysis. Bull. World Health Organ 65(5):663–737.
Kuhle S, Maguire B, Ata N, MacInnis N, Dodds L. 2017. Birth Weight for Gestational Age, Anthropometric Measures, and Cardiovascular Disease Markers in Children. J Pediatr 182:99–106.
Kumar A, Chaudhary K, Prasad S. 2010. Maternal indicators and obstetric outcome in the north Indian population: A hospital-based study. J Postgrad Med 56(3):192–195.
Kumar SN, Raisuddin S, Singh KJ, Bastia B, Borgohain D, Teron L, Sharma SK, Jain AK. 2020. Association of maternal determinants with low birth weight babies in tea garden workers of Assam. J Obstet Gynaecol Res 46(5):715–726.
Laflamme EM. 2010. Maternal hemoglobin concentration and pregnancy outcome: A study of the effects of elevation in EL alto, Bolivia. Mcgill J Med 13(1):47–55.
Long H, Yi JM, Hu PL, Li ZB, Qiu WY, Wang F, Zhu S. 2012. Benefits of iron supplementation for low birth weight infants: a systematic review. BMC Pediatr 16;12:99.
Longo LD. 1977. The biological effects of carbon monoxide on the pregnant woman, fetus, and newborn infant. AJOG 129(1):69–103.
Mahumud RA, Sultana M, Sarker AR. 2017. Distribution and determinants of low birth weight in developing countries. JPMPH 50(1):18–28.
Majumder PP. 1998. People of India: Biological diversity and affinities. Evol Anthropol 6(3):100–110.
Malik S, Ghidiyal RG, Udani R, Waingankar P. 1997. Maternal Biosocial Factors Affecting Low Birth Weight. Indian J. Pediatr 64(3):373–377.
Mammaro A, Carrara S, Cavaliere A, Ermito S, Dinatale A, Pappalardo EM, Militello M, Pedata R. 2009. Hypertensive disorders of pregnancy. J Prenat Med 3(1):1–5.
Mathew RJ, Bose A, Prasad JH, Muliyil JP, Singh D. 2014. Maternal periodontal disease as a significant risk factor for low birth weight in pregnant women attending a secondary care hospital in South India: A Case-control study. Indian J Dent Res 25(6):742–747.
Mavalankar DV, Gray RH, Trivedi CR. 1992. Risk factors for preterm and term low birthweight in Ahmedabad, India. Int J Epidemiol 21(2):263–272.
Mayor S. 2016. Low birth weight is associated with increased deaths in infancy and adolescence, shows study. BMJ 353:i2682.
McAnarney ER, Stevens-Simon C. 1990. Maternal Psychological Stress/Depression and Low Birth Weight: Is There a Relationship? Am J Dis Child 144(7):789–792.
McCormick MC. 1985. The Contribution of Low Birth Weight to Infant Mortality and Childhood Morbidity. N Engl J Med 312(2):82–90.
Mesleh R. 1986. The grand multipara-still an obstetric problem. J Obstet Gynaecol 7(2):84–87.
Metgud CS, Naik VA, Mallapur MD. 2012. Factors affecting birth weight of a newborn – a community based study in rural Karnataka, India. PLoS ONE 7(7).
Meyer JD, Nichols GH, Warren N, Reisine S. 2008. Maternal occupation and risk for low birth weight delivery: assessment using state birth registry data. J Occup Environ Med 50(3):306–15.
Misra DP. 1996. The effect of the pregnancy-induced hypertension on fetal growth: a review of the literature. Paediatr Perinat Epidemiol 10(3):244–63.
Miyake Y, Tanaka K, Okubo H, Sasaki S, Arakawa M. 2014. Alcohol consumption during pregnancy and birth outcomes: The Kyushu Okinawa Maternal and Child Health Study. BMC Pregnancy Childbirth 14(79).
Mohanty C, Prasad R, Srikanth Reddy A, Ghosh JK, Singh TB, Das BK. 2006. Maternal anthropometry as predictors of low birth weight. J Trop Pediatr 52(1):24–9.
Mondal B. 2000. Risk factors for low birth weight in Nepali infants. Indian J Pediatr 67(7):477–482.
Mumbare SS, Maindarkar G, Darade R, Yenge S, Tolani MK, Patole K. 2012. Maternal risk factors associated with term low birth weight neonates: a matched-pair case control study. Indian Pediatr 49(1):25–8.
Nair S, Rao RSP, Chandrashekar S, Acharya D, Bhat HV. 2000. Socio-demographic and maternal determinants of low birth weight: A multivariate approach. Indian J Pediatr 67(1):9–14.
Negandhi PH, Negandhi HN., Zodpey SP, Ughade SN, Biranjan JR. 2014. Risk factors for low birth weight in an Indian urban setting: A nested case control study. Asia Pac J Public Health 26(5):461–469.
Negi KS, Kandpal SD, Kukreti M. 2006. Epidemiological factors affecting low birth weight. JK Science 8(1):31–34.
Nelson SM, Telfer EE, Anderson RA. 2013. The ageing ovary and uterus: New biological insights. Human Reproduction Update 19(1):67–83.
Newton KP, Feldman HS, Chambers CD, Wilson L, Behling C, Clark JM, Molleston JP, Chalasani N, Sanyal AJ, Fishbein MH, Lavine JE, Schwimmer JB; Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN). 2017. Low and High Birth Weights Are Risk Factors for Nonalcoholic Fatty Liver Disease in Children. J Pediatr 187:141–146.e1.
O’Rahilly S, Farooqi IS. 2006. Genetics of obesity. Philosophical Transactions of the Royal Society B: Biol Sci 361(1471):1095–1105.
Offenbacher S, Katz V, Fertik G, Collins J, Boyd D, Maynor G, McKaig R, Beck J. 1996. Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol 67(10 Suppl):1103–13.
Omer H. 1986. Possible psychophysiologic mechanisms in premature labor. Psychosomatics 27(8):580–4.
Ozumba BC, Igwegbe AO. 1992. The challenge of grandmultiparity in Nigerian obstetric practice. J Obstet Gynaecol 37(4):259–264.
Patel A, Prakash AA, Das PK, Gupta S, Pusdekar YV, Hibberd PL. 2018. Maternal anemia and underweight as determinants of pregnancy outcomes: cohort study in eastern rural Maharashtra, India. BMJ Open 8:e021623
Patra J, Bakker R, Irving H, Jaddoe VW, Malini S, Rehm J. 2011. Dose-response relationship between alcohol consumption before and during pregnancy and the risks of low birthweight, preterm birth and small for gestational age (SGA)-a systematic review and meta-analyses. BJOG 118(12):1411–21.
Pivarnik JM. 1998. Potential effects of maternal physical activity on birth weight: Brief review. Med Sci Sports Exerc 30(3):400–406.
Pogodina C, Brunner Huber LR, Racine EF, Platonova E. 2009. Smoke-free homes for smoke-free babies: The role of residential environmental tobacco smoke on low birth weight. J Community Health 34(5):376–382.
Quigley ME, Sheehan KL., Wilkes MM, Yen SSC. 1979. Effects of maternal smoking on circulating catecholamine levels and fetal heart rates. J Obstet Gynaecol 133(6):685–690.
Radhakrishnan T, Thankappan KR, Vasan RS, Sarma PS. 2000. Socioeconomic and demographic factors associated with birth weight: a community based study in Kerala. Indian pediatr 37(8):872–6.
Raje S, Rao S. 2015. Maternal Food Consumption Patterns and Risk of Low Birth Weight in Rural Maharashtra. Indian J Nutr Diet 52(2):153–165.
Rao S, Gokhale M, Joshi S, Kanade A. 2010. Early life undernutrition and adolescent pregnancy outcome in rural India. Ann Hum Biol 37(4):475–87.
Rasmussen KM, Yaktine AL editors. 2009. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington (DC): National Academies Press (US).
Resnik R. 2002. Intrauterine growth restriction. J Obstet Gynecol 99(3):490–496.
Rijken MJ, McGready R, Boel ME, Poespoprodjo R, Singh N, Syafruddin D, Rogerson S, Nosten F. 2012. Malaria in pregnancy in the Asia-Pacific region. Lancet Infect Dis 12(1):75–88.
Rondó PH, Ferreira RF, Nogueira F, Ribeiro MC, Lobert H, Artes R. 2003. Maternal psychological stress and distress as predictors of low birth weight, prematurity and intrauterine growth retardation. Eur J Clin Nutr 57(2):266–72.
Roy S, Motghare DD, Ferreira AM, Vas FS, Kulkarni MS. 2009. Maternal determinants of low birth weight at a tertiary care. J Fam Welf 55(1):79–83.
Saili A. 2008. Essential care of low birth weight neonates. Indian pediatr 45(1):13–5.
Sallout B, Walker M. 2003. The fetal origin of adult diseases. J Obstet Gynaecol 23(5):555–560.
De Santis M, De Luca C, Mappa I, Spagnuolo T, Licameli A, Straface G, Scambia G. 2012. Syphilis Infection during pregnancy: fetal risks and clinical management. Infect Dis Obstet Gynecol 2012:430585.
Sarkar NN. 2008. The impact of intimate partner violence on women’s reproductive health and pregnancy outcome. J Obstet Gynaecol 28(3):266–271.
Scholl TO, Salmon W, Miller LK. 1986. Smoking and adolescent pregnancy outcome. J Adolesc Health Care 7(6):390–4.
Seidman DS., Dollberg S, Stevenson DK, Gale R. 1991. The effects of high parity and socioeconomic status on obstetric and neonatal outcome. Arch Gynecol Obstet 249(3):119–127.
Sen J, Roy A, Mondal N. 2009. Association of maternal nutritional status, body composition and socio-economic variables with low birth weight in India. J Trop Pediatr 56(4):254–259.
Seth R, Bose V, Qaiyum Y, Chandrashekhar R, Kansal S, Taneja I, Seth T. 2018. Social Determinants of Child Marriage in Rural India. Ochsner J 18(4):390–394.
Shah PS. 2010. Parity and low birth weight and preterm birth: A systematic review and meta-analyses. Acta Obstet Gynecol Scand 89(7):862–875.
Shan D, Qiu PY, Wu YX, Chen Q, Li AL, Ramadoss S, Wang RR, Hu YY. 2018. Pregnancy Outcomes in Women of Advanced Maternal Age: a Retrospective Cohort Study from China. Sci Rep 16;8(1):12239.
Shankar H, Kumar N, Sandhir R, Singh MP, Mittal S, Adhikari T, Tarique M, Kaur P, Radhika MS, Kumar A, Rao DN. 2019. Association of dietary intake below recommendations and micronutrient deficiencies during pregnancy and low birthweight. J Perinat Med 25;47(7):724–731.
Sheen JJ, Wright JD, Goffman D, Kern-Goldberger AR, Booker W, Siddiq Z, D’Alton ME, Friedman AM. 2018. Maternal age and risk for adverse outcomes. Am J Obstet Gynecol 219(4):390.e1-390.e15.
Shivakumar N, Dwarkanath P, Bosch R, Duggan C, Kurpad AV, Thomas T. 2018. Influence of gestational weight gain on low birth weight in short-statured South Indian pregnant women. Eur J Clin Nutr 72(5):752–760.
Shrivastava J, Agrawal A, Giri A. 2016. Maternal anthropometry in relation to birth weight of newborn: A prospective hospital based study. Indian J Child Health 03(01):59–63.
Singh G, Chouhan R, Sidhu K. 2009. Maternal factors for low birth weight babies. Med J. Armed Forces India 65(1):10–12.
Sreeramareddy CT., Shidhaye RR, Sathiakumar N. 2011. Association between biomass fuel use and maternal report of child size at birth – An analysis of 2005-06 India Demographic Health Survey data. BMC Public Health 11(1):1–10.
Stangret A, Skoda M, Wnuk A, Pyzlak M, Szukiewicz D. 2017. Mild anemia during pregnancy upregulates placental vascularity development. Med Hypotheses 102:37–40.
Subramanyam MA, Ackerson LK, Subramanian SV. 2010. Patterning in birthweight in India: Analysis of maternal recall and health card data. PLoS ONE 5(7):p.e11424.
Tabcharoen C, Pinjaroen S, Suwanrath C, Krisanapan O. 2009. Pregnancy outcome after age 40 and risk of low birth weight. J Obstet Gynecol 29(5):378–383.
Tan EK, Tan EL. 2013. Alterations in physiology and anatomy during pregnancy. Best Pract Res Clin Obstet Gynaecol 27(6):791–802.
Tanbo TG, Bungum L. 1987. The grand multipara-maternal and neonatal complications. Acta Obstet Gynecol Scand 66(1):53–6.
Tellapragada C, Eshwara VK, Bhat P, Acharya S, Kamath A, Bhat S, Rao C, Nayak S, Mukhopadhyay C. 2016. Risk Factors for Preterm Birth and Low Birth Weight Among Pregnant Indian Women: A Hospital-based Prospective Study. J Prev Med Public Health 49(3):165–75.
Vats H, Saxena R, Sachdeva MP, Walia GK, Gupta V. 2021. Impact of maternal pre-pregnancy body mass index on maternal, fetal and neonatal adverse outcomes in the worldwide populations: A systematic review and meta-analysis. Obes Res Clin Pract 15(6):536–545.
Veena SR, Kumaran K, Swarnagowri MN, Jayakumar MN, Leary SD, Stein CE, Cox VA, Fall CH. 2004. Intergenerational effects on size at birth in South India. Paediatr Perinat Epidemiol 18(5):361–70.
Velankar DH. 2009. Maternal Factors Contributing to Low Birth Weight Babies in an Urban Slum Community of Greater Mumbai. Bombay Hosp J 51(1):26–35.
Visentin S, Grumolato F, Nardelli GB, Di Camillo B, Grisan E, Cosmi E. 2014. Early origins of adult disease: low birth weight and vascular remodeling. Atherosclerosis 237(2):391–9.
Visintin C, Mugglestone MA, Almerie MQ, Nherera LM, James D, Walkinshaw S; Guideline Development Group. 2010. Management of hypertensive disorders during pregnancy: summary of NICE guidance. BMJ 25;341:c2207.
Ward C, Lewis S, Coleman T. 2007. Prevalence of maternal smoking and environmental tobacco smoke exposure during pregnancy and impact on birth weight: retrospective study using Millennium Cohort. BMC Public Health 16,7:81.
Wjst M, Popescu M, Trepka MJ, Heinrich J, Wichmann HE. 1998. Pulmonary function in children with initial low birth weight. Pediatr Allergy Immunol 9(2):80–90.
Wollmann HA. 1998. Intrauterine growth restriction: Definition and etiology. Horm Res 49(2 SUPPL.)1–6.
World Health Organisation. 2004. ICD-10 : international statistical classification of diseases and related health problems : tenth revision. 2nd ed. World Health Organisation.
World Health Organization. 2005. Alcohol, gender and drinking problems in low and middle income countries. World Health Organization.
World Health Organisation. 2011. Antepartum Haemorrhage Green-top Guideline No. 63.
World Health Organisation. 2016. Recommendations on antenatal care for a positive pregnancy experience.
Wynn AHA, Crawford MA, Doyle W, Wynn SW. 1991. Nutrition of Women in Anticipation of Pregnancy. Nutr Health 7(2):69–88.
Xiao PL, Zhou YB, Chen Y, Yang MX, Song XX, Shi Y, Jiang QW. 2015. Association between maternal HIV infection and low birth weight and prematurity: a meta-analysis of cohort studies. BMC Pregnancy Childbirth 8;15:246.
Young MF, Nguyen PH, Addo OY, Hao W, Nguyen H, Pham H, Martorell R, Ramakrishnan U. 2015. The relative influence of maternal nutritional status before and during pregnancy on birth outcomes in Vietnam. Eur J Obstet Gynecol Reprod Biol 194:223–7.
Zhang Q, Li Z, Ananth CV. 2009. Prevalence and risk factors for anaemia in pregnant women: A population-based prospective cohort study in China. Paediatr Perinat Epidemiol 23(4):282–291.
Zhang X, Platt RW, Cnattingius S, Joseph KS, Kramer MS. 2007. The use of customised versus population-based birthweight standards in predicting perinatal mortality. BJOG: Int. J. Obstet. Gynaecol 114(4):474–477.
Zhou Y, Li H, Zhang Y, Zhang L, Liu J, Liu J. 2019. Association of Maternal Obesity in Early Pregnancy with Adverse Pregnancy Outcomes: A Chinese Prospective Cohort Analysis. Obesity (Silver Spring) 27(6):1030–1036.


Received: 04.02.2022; Revised: 05.12.2022; Accepted: 05.12.2022