Anthropological Review Vol. 85(2), 13–62 (2022)
Anthropological Review
Available online at: https://doi.org/10.18778/1898-6773.85.2.02
Sacral Spina Bifida Occulta: A Frequency Analysis of Secular Change
Ella R Kelty*
Anatomical Sciences Unit of the School of Biomedicine, Adelaide University, Adelaide, Australia
Department of Archaeology, Flinders University, Adelaide, Australia
Maciej Henneberg
Anatomical Sciences Unit of the School of Biomedicine, Adelaide University, Adelaide, Australia
Department of Archaeology, Flinders University, Adelaide, Australia
Institute of Evolutionary Medicine, University of Zurich, Zurich, Switzerland
ABSTRACT: Substantial relaxation of natural selection beginning around 1900 changed the mutation/selection balance of modern genetic material, producing an increase in variable anatomical structures. While multiple structures have been affected, the temporal increase in variations of the sacrum, specifically, ‘Sacral Spina Bifida Occulta,’ have been reliably demonstrated on a localised scale. Calculation of largescale frequency has been hindered by the localised nature of these publications, the morphological variability of this variation, and potential pathological associations, which have produced divergent classifications, and conflicting reported rates of occurrence. A systematic review of the reported literature was conducted to provide an objective analysis of Sacral Spina Bifida Occulta frequency from 2500 BCE to the present. This review was designed to compensate for observed inconsistencies in reporting and to ascertain, for the first time, the temporal trajectory of this secular trend. A systematic review of Sacral Spina Bifida Occulta literature was conducted through the strict use of clinical meta-analysis criteria. Publications were retrieved from four databases: PubMed, Embase, the Adelaide University Library database, and Google Scholar. Data were separated into three historical groups, (1 = <1900, 2 = 1900 to 1980 and 3 = >1980), and frequency outcomes compared, to determine temporal rates of occurrence.
A total of 39/409 publications were included in the final analysis, representing data for 16,167 sacra, spanning a period of 4,500 years. Statistically significant results were obtained, with total open S1 frequency increasing from 2.34%, (79 to 1900CE), to 4.80%, (1900 to 1980CE) and to 5.43% (>1980CE). These increases were significant at p<0.0001, with Chi-squared analysis. A clear secular increase in the global frequency of Sacral Spina Bifida Occulta has been demonstrated from 1900 to the present. This research provides a novel and adaptable framework for the future assessment of variation distribution, with important implications for the fields of biological anthropology and bioarchaeology.
KEY WORDS: Sacral Spina Bifida Occulta (SSBO), frequency, classification, natural selection
Abbreviations: Sacral Spina Bifida Occulta (SSBO), Spina Bifida Cystica (SBC), Neural Tube Defect (NTD)
Introduction
The relaxation of natural selection can be attributed to the decreased rate of infant mortality and the increased rate of adult survivability from 1900 onwards (Ulizzi et al. 1998). These changes were shaped by improved clinical understanding of disease, the invention of increasingly effective medication, and an improvement in prenatal and postnatal medical care (Ruhli and Henneberg 2013; Solomon et al. 2009). Consequently, survivorship to the age of reproduction (15 years) increased from <50% in 1850 to slightly >90% by 1900 (Greene 2001). Overall, the probability that an average person born into a population will be able to pass their genes to the next generation rose from 0.30 to 0.95 (Saniotis and Henneberg 2011). This reduction in the opportunity for selection, altered the mutation/selection balance which precipitated phenotypic variation (Cairnes and Gariepy 1990; Lee et al. 2011). Such an increase has been observed in a number of modern physiological, immunological, and morphological characteristics, the most well-known of which is the increase in lactose intolerance and the congenital absence of the third molar (Ingram et al. 2009; Swallow 2003). Occurring over a relatively short period of evolutionary history, (120 years), these modern secular changes have been observed in multiple correlating anatomical structures.
One such example includes increases observed in the retention of the embryonic variant, the median artery. This embryonic vessel typically regresses at 8 weeks gestation, but retention of this artery into adulthood has experienced an increase of 20%, over a period of the last 170 years (Lucas et al. 2020). The atypical fusion of one or more tarsal bones of the foot has also been subject to observed increases after 1900, with an increase of >12%, evidenced over a period of 50 years (Ruhli et al. 2003). The timing of these changes in anatomical structures, coincides with observed increases in sacral variations, most specifically the ‘vertebral anomaly’ Sacral Spina Bifida Occulta, (SSBO).
Sacral Spina Bifida Occulta (SSBO) is a condition difficult to define due to the variability with which it is described in the literature, and the broad spectrum of defects this condition can represent (Albrecht et al. 2007; Eubanks and Cheruvu 2009). SSBO is often considered the mildest manifestation of Neural Tube Defect (NTD), specifically of the highly debilitating Spina Bifida Cystica (SBC), which has been identified as the most common congenital anomaly of the 21st century (Kallen and Lofkvist 1984; Morrison et al. 1998). Characterised skeletally, by the absence or non-fusion of one or multiple posterior vertebral arches, SSBO variably includes deformation of the laminae, neural arch, or pedicles of vertebrae (Post 1966; Sutow and Pryde 1955). While this anomaly can occur at any level of the vertebral column, the malformation of the last lumbar vertebra and the first sacral vertebra is the most routinely observed, studied, and reported (Sairyo et al. 2006). Due to the severity of deformation caused by SSBO, (which typically presents as the exposure of the sacral canal, or absence of the dorsal wall), this condition is easily identifiable in dry human sacra, and can be reliably distinguished from post-depositional erosion or damage, (Figs 1, 2 and 3). Therefore, observations of this condition in dry human sacra are reliable, and publications which provide frequency data in this context can be assumed to be accurate and objective.

Fig. 1. Dry human sacrum with a typically formed dorsal wall – fully fused sacral vertebrae. (Photograph taken by lead author (Kelty 23/09/2021). Specimen B53 from St Marys archaeological collection, ethically held by The University of Adelaide).

Fig. 2. Dry human sacrum demonstrating ‘Total SSBO’ or non fusion of arches of all sacral segments. (Photograph taken by lead author (Kelty 23/09/2021). Specimen B79 from St Marys archaeological collection, ethically held by The University of Adelaide).
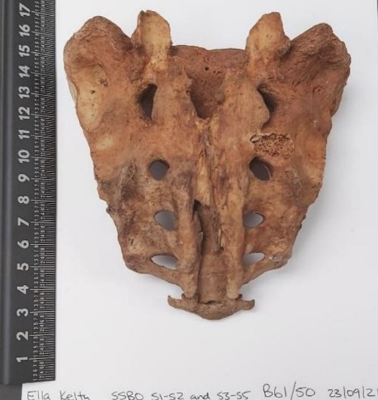
Fig. 3. Dry human sacrum demonstrating non-fusion of sacral segments, S1 and S4-S5. The most commonly observed configuration of S1 non-fusion. (Photograph taken by lead author (Kelty 23/09/2021). Specimen B61 from St Marys archaeological collection, ethically held by the University of Adelaide).
Deformation at all levels of the sacrum can occur with varying degrees of regularity, dependent on the sacral segment involved. The most common observation of non-fusion occurs at segments S4-S5, which can reach upwards of 90% of individuals with European ancestry (Fidas et al. 1987). Thus, this deformation pattern is clinically recognised as a natural morphological variation, termed the sacral hiatus (Abera et al. 2021; Henneberg and Henneberg 1999). Deformations of segments S2 and S3 have lower frequencies, (1% to 10%), but to date are also considered natural variations, due to their sometimes inclusion into the hiatus (Simriti et al. 2017). This inclusion of S2-S5 in the natural variation of the sacrum suggests that these specific patterns of deformation have no pathological associations and are therefore of no clinical importance (Kumar and Tubbs 2011).
Non-fusion of the first sacral segment usually has a lower frequency, similar to that of S2 and S3, but is unreliably reported, with estimations ranging from 8% (Piontek 1971) to 23% (Sairyo et al. 2006). Unlike the segments S2-S5, S1 has clear pathological associations having been reliably correlated with enuresis, posterior disk herniation, and lower back pain (Eubanks and Cheruvu 2009; Sutow and Pryde 1955). Non-fusion of sacral segments inclusive of S1 can be considered morphologically and clinically important, irrespective of non-fusion or fusion of other segments. It is for this reason that Sacral Spina Bifida Occulta can be specifically defined as non-fusion inclusive of the first sacral segment (Henneberg and Henneberg 1999; Lee et al. 2011; Solomon et al. 2009).
The Pelvis (Os Coxae) is the most variable aspect of the human skeleton due to its high levels of sexual dimorphism, with the sacrum being considered the most variable aspect within that structure (Steyn and Iscan 2008). Variations to the structure of the sacral canal can also influence variation in the resulting morphology of the surrounding Os Coxae (Kurki 2013). The degree to which these variations can influence pregnancy, birth, overall health, and forensic sex identification, has resulted in a relative wealth of clinical, anthropological, and archaeological assessments of this variation over the last century (Henneberg and Henneberg 1999). The identification and classification of Sacral Spina Bifida Occulta was first described in the anthropological literature by Willis (1923). Willis popularised the characterisation of this condition as a ‘vertebral anomaly’ of no clinical significance, recording only a 1.2% frequency in 748 historical subjects (Willis 1923). Anthropological interest in SSBO was shaped by this definition but was characterised by inconsistency in reported frequencies. Ferembach (1963) famously reported a 76% frequency in a sample from 12,500 BCE, but this was hard to substantiate, due to the small sample size and the 8% to 23% occurrence which typified the literature of this period. Inconsistencies in reported SSBO frequencies were further exasperated by the clinical recognition of this condition, which aligned with investigations of neural tube defects in 1980 and introduced a new generation of conflicting classifications and frequency calculation methods (Molloy et al. 2017; Scatliff et al. 2013).
Investigations into the temporal increase of SSBO frequency in the modern era, and its correlation to the relaxation of natural selection around 1900, have ultimately been impeded by the number of academic debates, controversies and disagreements which characterise this research area (Shore 1930; Zemirline et al. 2013). A long-standing consensus within the medical community that anatomically modern humans are no longer evolving under the operation of natural selection, has prevented largescale research into these changing anatomical structures and their potential impact on the health of future populations (Kumar and Singh 2003; Rühli and Henneberg 2013). While small scale and localised studies have been conducted which reliably support this correlation between various changing modern anatomical structures and the relaxation of natural selection in the industrialised world from 1900 onwards, (Lucas et al. 2020; Rühli et al. 2003) large-scale assessments and widespread acceptance of this phenomenon have yet to be established.
This observed lack of academic consensus has prevented reliable calculation of SSBO frequency over time, which is additionally impeded by the small number of publications which contain reliable data for this condition (Zemirline et al. 2013). These inherent limitations have been addressed by modern SSBO research, which provides more reliable assessments of frequency than historically observed (Kumar and Singh 2003). Interestingly, an 11% frequency of this condition was observed in Pompeii (79CE, Henneberg and Henneberg 1999), being about one half of modern European assessments of about 20% (Saluja 1988). This led Henneberg and Henneberg (1999) to suggest that a secular and microevolutionary trend could be observed in SSBO frequency. This increase was further substantiated by Solomon et al. (2009) and Lee et al. (2011), who demonstrated an increase in the frequency of SSBO at S1 in Australian and European birth cohorts, from 1940s to 1980s. This is interesting, as these localised studies demonstrate an increase that not only correlates with the observed relaxation of natural selection around 1900, but that also coincides with relative increases in similar anatomical variations during the same period. It is therefore hypothesised that the generation of a large-scale, geographical, and temporal assessment of SSBO frequency will produce evidence of a clear secular trend in the increase of this condition from 1900 onwards.
Materials and Methods
A literature review was performed to collect all available publications pertaining to SSBO frequency as previously defined. This review generated a total of 409 foundational or peer-reviewed publications. Predetermined exclusion criteria were used to determine the relevance of each publication and assess the quality of their reported segmentation data (Fig. 4). In total 39 of 409 (<10%) publications were included in the frequency analysis, producing a total sample size of 16,167 sacra, which spanned 25 international regions (Fig. 5) and a period of 4,500 years. Male and female sample sizes were also recorded where reported, with a total male sample size of 3,992 and female sample size of 3,818, with 8,357 (51.69% of 16,167) having undesignated sex.
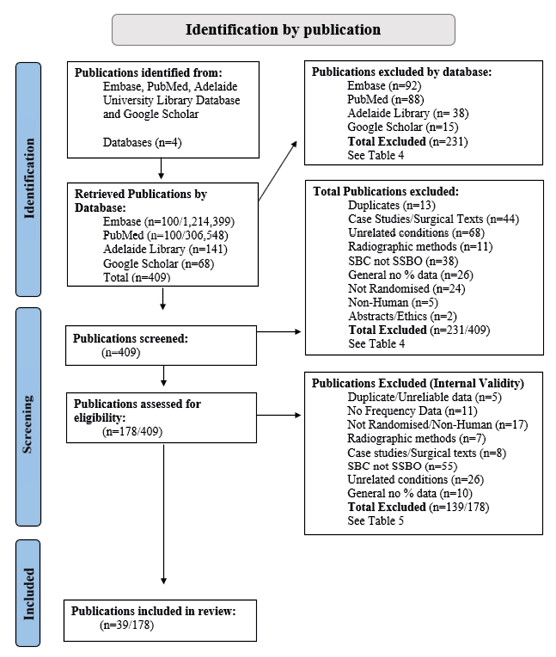
Fig. 4. PRISMA Flow chart of literature analysis method, with added inclusion/exclusion criteria.
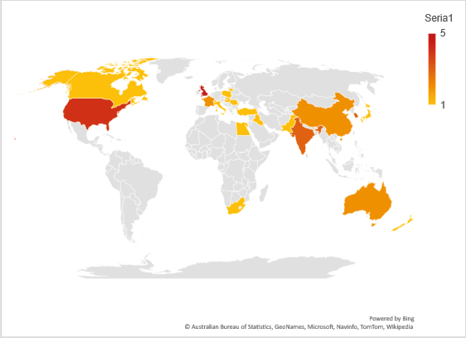
Fig. 5. World map showing distribution of included SSBO data for the literature analysis. Country of origin and number of publications per country included.
In order to reliably evaluate the true frequency of SSBO it was necessary to design a method that could enable the review of all available and relevant literature, while overcoming observed inconsistencies in classification and frequency calculation. It was also imperative to demonstrate that modern human skeletal anatomy is subject to evolutionary change, and that increases in SSBO frequency directly correlate with the recent relaxation of selection shift. The literature was collected, assessed, and analysed according to strict clinical meta-analysis guidelines to ensure that data were reviewed systematically (Balduzzi et al. 2019; Page et al. 2021). As this research does not contain clinical trials or patients, some meta-analysis criteria could not be applied, and the decision was made to conduct a quantitative literature review/frequency analysis instead. To guarantee cohesion, validity and accuracy within the research design, all analyses were conducted according to the requirements of a meta-analysis where possible (Higgins et al. 2003; Page and Moher 2017).
Literature Review Method
Publications for the literature review were collected through the use of Embase, PubMed, Google Scholar, and the University of Adelaide’s library database. Embase and PubMed were used primarily for the collection of clinical literature relating to SSBO and did not include anthropological and archaeological data for this condition, particularly not from the early 20th century. Google Scholar was used as a means to find those publications not available in the medical literature databases, and the University of Adelaide Library was used to gain access to those publications found in Google Scholar that were restricted by paywalls. The Adelaide University Library database was the most practical resource to use for supplementary access to these publications, as both authors are members of The School of Biomedicine at this University.
Databases were searched for keywords; Sacral Spina Bifida Occulta, Spina Bifida Occulta, Neural Tube Defect, Spina Bifida and Occult Spinal Dysraphism. Keywords were supplemented by corresponding searches for, incidence, prevalence, frequency, and rates. Results from each database underwent two rounds of screening, the initial publication screening, (12/2/2021 – 03/05/2021) and the internal validity screening, (13/09/2021 – 2/10/2021), which included different criteria. The initial publication screening was conducted through a process of examining the abstract, results and conclusions of each publication, and including/excluding each publication based on a set of predetermined exclusion criteria (Fig. 4) (Balduzzi et al. 2019). Publications that were included through the initial publication screening were then analysed a second time with more stringent predetermined exclusion criteria, which focussed on the assessment of the internal validity of each study (Page et al. 2021).
Different retrieval strategies were used dependent on the database. PubMed and Embase are clinical databases that were used to source potential clinical data on the frequency of SSBO. Due to the volume of publications generated from such expansive databases, as a result of the search strategies outlined (Table 1), only the top 100 search results were included for screening. The University of Adelaide library and Google scholar databases were used primarily to retrieve anthropological and archaeological data on the frequency of this condition. Due to the nature of these databases, specific search strategies were not used, however, each afore mentioned key word was searched, and any relevant publications were retrieved. This was further complemented by the use of these databases to retrieve publications cited in already analysed works and to expand upon the key words to include, sacral hiatus, paleoepidemiology, sacral anomaly, sacral deformity and osteoarchaeology.
It should be noted that due to the nature of SSBO and its presence in historical and archaeological populations, this review of the literature is amalgamating already published data on the frequency of this condition in dry human sacra, from cadaver studies and through anonymised radiographic data. Therefore, this review is bio-anthropological and does not include patients, clinical trials, medical equipment, additional reviewers, ethics approval or funding grants. All publications were reviewed by the lead author manually, no automation process or equipment was used, and no additional reviewers or external parties were involved.
Table 1. Search strategies for publication retrieval from each database
| Database | Keywords | Search String* | Retrieved/Generated* |
| PubMed | Sacral Spina Bifida Occulta, Spina Bifida Occulta, Neural Tube Defect, Spina Bifida and Occult Spinal Dysraphism. + Incidence, Prevalence, Frequency, and Rates |
(Sacral Spina Bifida Occulta) OR (Spina Bifida Occulta) OR (Neural Tube Defects) OR (Spina Bifida) OR (Occult Spinal Dysraphism) AND (Incidence) OR (Rates) OR (Frequency) OR (Prevalence) | 100/ 306,548 |
| Embase | Sacral Spina Bifida Occulta, Spina Bifida Occulta, Neural Tube Defect, Spina Bifida and Occult Spinal Dysraphism. + Incidence, Prevalence, Frequency, and Rates |
Exp spinal dysraphism / or exp neural tube defects / or exp open spinal dysraphism / and exp incidence / or exp frequency / or exp prevalence / | 100/ 1,214,399 |
| The University of Adelaide | Sacral Spina Bifida Occulta, Spina Bifida Occulta, Neural Tube Defect, Spina Bifida and Occult Spinal Dysraphism. + Incidence, Prevalence, Frequency, and Rates + Sacral Hiatus, Paleoepidemiology, Sacral Anomaly, Sacral Deformity and Osteoarchaeology |
N/A | 141 |
| Google Scholar | Sacral Spina Bifida Occulta, Spina Bifida Occulta, Neural Tube Defect, Spina Bifida and Occult Spinal Dysraphism. + Incidence, Prevalence, Frequency, and Rates + Sacral Hiatus, Paleoepidemiology, Sacral Anomaly, Sacral Deformity and Osteoarchaeology |
N/A | 68 |
*Search string and number of publications generated are not applicable to the University of Adelaide Library and Google scholar databases.
Initial publication screening parameters
Once all 409 publications had been retrieved, they underwent the initial screening process and were included/excluded based on stringent predetermined criteria, (Fig. 4). As the primary objective of this literature analysis was to obtain frequency data for SSBO, publications that did not include frequency data for this condition, data for SSBO specifically, or those that did not include random samples, were excluded. Case studies which discussed only single examples and therefore had no frequency data were excluded. Surgical texts were also excluded on the basis that they related to the diagnosis, management, treatment, and surgical outcomes of spinal dysraphism. As such the frequency of occurrence was not reported, as all individuals observed had already been diagnosed with this condition. A singular list of abstracts for a conference on the neurosurgical management of spinal dysraphism was also excluded due to lack of detailed frequency data. Publications detailing novel radiographic methods for the identification of SSBO were also excluded, as prevalence data were not reported. One publication pertained solely to ethics, one was in reference to widescale arsenic poisoning, and a number were related to non-human clinical trials which were of no relevance to this research.
‘General SSBO’ included publications that were designed as informative documents on the identification, diagnosis, and treatment of SSBO from a clinical perspective. These publications did not include frequency data, and more than half were in reference to SBC not SSBO. This misidentification of SSBO as the neural tube defect SBC, was also an exclusion criterion. A number of publications retrieved from Embase, and PubMed also included publications on pathologies completely unrelated to SSBO. Issues in identifying SSBO data specifically, were further complicated by the number of associated pathologies researched clinically. Publications relating to these pathologies, were also assessed, and included only if the frequency data were wholly separated from those of the associated pathology, and if adequate and appropriate control groups were used (Page and Moher 2017; Page et al. 2021).
A disproportionate number of the retrieved publications were related to Spina Bifida Cystica (SBC) and Neural Tube Defects, and included no reference to, or data for SSBO. This was the consequence of an early proposal to compare frequency data for these two conditions to ascertain the importance of their relative patterns of occurrence. As this research progressed, it was determined that SBC frequency was already reliably established in the literature, and therefore these studies were not included in the final analysis (Fig. 4). Trusted data for SBC, however, were obtained from national and global birth registers, derived from these excluded publications, to compare the relative prevalence of this condition with that of SSBO for the same period (Atta et al. 2016). This was achieved through the calculation of mean values for reported births with SBC per 1000, in European populations. These are not included in the results but were generated for the purpose of aiding the discussion.
Internal Validity Screening Parameters
Once these publications had been screened for the more basic parameters, (inclusion of SSBO frequency data), the remaining 178 publications were subjected to an additional, more stringent, screening process, to access the internal validity and address the risk of bias in their results (Higgins et al. 2003). The quality of included segmentation data was assessed, and those that did not include data for deformation of S1 specifically, or which reported duplicate data, were excluded. Archaeological and anthropological texts from the early 20th century which included purely textual anecdotes, were thoroughly scrutinised to ensure that sample sizes and case numbers were accurate and did not contain any missing or unclear data. Any uncertainty as to the clarity, totality, or accuracy of the data from these publications resulted in them being excluded to ensure the generation of meaningful and reliable results (Higgins et al. 2003). Publications that included duplicate data already screened in previous publications, or data that could not be reliably differentiated from other osteological assessments from similar or sometimes the same archaeological sites, were also excluded.
Those publications which assessed the association between SSBO, and a range of pathologies were also assessed to ensure that frequency data for SSBO were wholly separable from those of the associated pathology, and that adequate control groups had been used. Those which did not provide adequate control groups, or studies which included only patients with a pathology, or deformity, reliably associated with SSBO, (eg: Cutaneous stigmata), were excluded on the basis that they did not represent the true frequency of this condition. Radiological assessments of this condition, which focussed on novel methods for the identification of SSBO, were also excluded if they contained zero or duplicate frequency data for this condition, or if the level of deformity, (segment), was not reported.
Once completed, this review of the current literature produced 39/409 publications for inclusion into the frequency data analysis. The included publications, as outlined in detail in the appendix, ranged in publication date from 1932 to 2019, 30 of these were peer reviewed, with the remaining nine having been published before the introduction of the peer review system. All included publications reported SSBO frequency data that were analysed and deemed reliable, and no publication was assessed which examined the frequency of SSBO and did not produce at least one case of this condition.
Frequency Analysis Methods
A total of 39/409 publications were included in the final frequency analysis having conformed to the outlined inclusion criteria, (Fig. 4). Numbers were allocated to each publication and citation, location and dating details were recorded for each. Reported case numbers of identified SSBO were divided by reported sample sizes, and multiplied by 100, to produce percentage values. This was completed for each possible combination of reported deformation, across all sacral segments. This included deformation of segments inclusive of S1, (ie: L5-S1, S1andS5), and calculation of male and female frequencies (Henneberg and Henneberg 1999; Lee et al. 2011; Solomon et al. 2009).
Recalculations were made where reported prevalence was not clearly presented, with some cases and sample sizes being combined where necessary, (control/patient and multiple juvenile samples). Patient groups that were proven to be random (not commonly or primarily associated with SSBO) were combined with control group sample sizes, and case numbers, to determine frequency for the whole group. Publications that separated subadults (1–15 years) into smaller sub-divisions of age, (eg: 1–2 years, 3–4 years etc.) were also grouped together, and an identical method was used to determine the relative frequency (Page et al. 2021). Similar additions were also made with the male and female frequency calculations. This occurred where male and female cases were recorded for both the control and patient groups, which were then combined to determine the frequency, as per the method outlined above. Instances where sex was separated into age categories, of girl/boy, female/male structure, were also combined to determine the frequency by sex (Fidas et al. 1987).
Once this information had been collected for all 39 publications, the resulting data were separated into three distinct historical groups. This was done to consider the 4,500-year time span, to test the hypothesised increase of this condition after 1900 and 1980 and to ensure that each study would be accurately weighted. This separation was determined according to calculated date of birth of each group. Birth dates were either used as reported in more modern publications or estimated by subtracting average life expectancy figures from burial dates for historical collections (WHO 2012; WHO 2020). Historical Group 1 (HG1) included date range 2,500BCE to 1,900CE, the second Historical Group (HG2) encompassed all material dating from 1,900 to 1980CE while Historical Group 3 (HG3) included the remaining data for the period 1980CE to 2020CE. Male and female frequency data, where available, were also separated into historical groups, although an absence of reported sex data for HG3 did affect the results of this group.
Statistical methods
While data for SSBO were recorded for each sacral segment, only data for deformation inclusive of S1 were included into the statistical analysis. Total sacra observed, and total number of cases were determined for each historical group. Contingency tables were generated in the SSPS.25 software, (Tables 2 and 3) and Chi-squared calculation with Yates’s correction, and corresponding p-values, were used to assess the direction of effect for these three groups (Henneberg and Henneberg 1999; Lee et al. 2011; Solomon et al. 2009). The available male and female data for all three historical groups were treated in the same way.
All statistical calculations were performed using the SSPS.25 software by the primary author with instruction and assistance from the secondary author. No external resources were used to complete this analysis and no additional reviewers were integrated into the assessment.
Table 2. Contingency table used to generate Chi-Squared statistic for the total frequency of SSBO
| Historical Group 1 <1900 |
Historical Group 2 1900-1980 |
Historical Group 3 >1980 |
|
| Total Sacra Observed | 6,901 | 8,074 | 1,192 |
| Total SSBO Cases Identified | 922 | 1,503 | 281 |
Total frequencies determined for comparison of Historical Groups 1 and 2, 2 and 3 and 1 and 3.
Table 3. Contingency table used to generate Chi-Squared statistic for the male/female frequencies of SSBO
| Historical Group 1 <1900 |
Historical Group 2 1900-1980 |
Historical Group 3 >1980 |
|
| Total Male Sacra Observed | 790 | 2,883 | 319 |
| Total Male Cases of SSBO | 98 | 738 | 46 |
| Total Female Sacra Observed | 720 | 2,830 | 268 |
| Total Female Cases of SSBO | 69 | 458 | 74 |
Male and Female frequencies determined through comparison of Historical Groups 1 and 2, 2 and 3 and 1 and 3.
Results
A total of 39 publications were included from the 409 retrieved and screened during the literature review process (Fig. 4). The results of both screening processes, with exclusion/inclusion criteria outlined for each publication, throughout each process, are presented in the appendix. Citation details and exact frequency data collected from each included study are also included in the appendix.
The frequency analysis results demonstrated a clear and statistically significant increase in the frequency of SSBO after 1900 (Table 4 and Fig. 6). The calculation of the total frequencies for historical groups one and two, evidenced a 5.25% increase in SSBO frequency, (<1900 to 1980), Chi-squared 54.503 (p<0.0001). The comparison of total frequencies between historical groups two and three also provided a very statistically significant result, with a 4.98% increase, Chi-squared 10.543 (p<and a p-value of <0.0012). Total frequency comparison was also completed between historical groups one and three, which demonstrated an increase of 10.23% from the period <1900, to the present, Chi-squared 57.843 (p<0.0001).
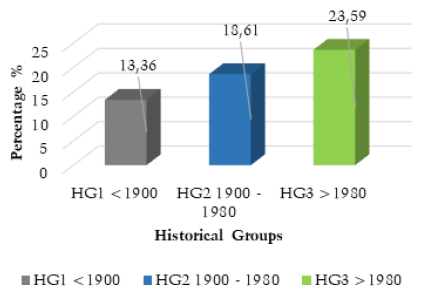
Fig. 6. Total SSBO frequency by historical group. Data derived from numerical analysis of included literature and frequency calculations outlined in Table 4.
Table 4. Total data included in each historical group
| Historical Group 1 <1900 |
Historical Group 2 1900-1980 |
Historical Group 3 >1980 |
|
| Total Included Publications | 18 | 17 | 7 |
| Total Included Studies | 28 | 40 | 7 |
| Total Sacra Observed | 6,901 | 8,074 | 1,192 |
| Total SSBO Cases Identified | 922 | 1,503 | 281 |
| Total Frequency | 13.36% | 18.61% | 23.59% |
| Total Increase | 2.40% | 5.25% | 4.98% |
| Chi-Square Value | HG1-HG2 54.503 |
HG2-HG3 10.543 |
HG1-HG3 57.843 |
| p-Value | <0.0001% | <0.0012% | <0.0001% |
Detailed representation of the total data for each historical group includes significance calculations and data used to calculate frequency.
The male and female frequency calculations for the three historical groups also produced statistically significant results for an increase of SSBO after 1900 and 1980. A clear increase was demonstrated in males (13.19%) and females (6.60%) for historical groups one and two, generating Chi-squared values of 40.618 and 14.737 (for both p<0.0001). A decrease was observed in male frequency of -11.17%, (Chi-squared=12.209, p<0.0005) between historical groups two and three, but an increase was demonstrated for females (Chi-squared = 14.105, p<0.0002). (Table 5 and Fig. 7).
Table 5. Male and female data included by historical group
| Historical Group 1 <1900 |
Historical Group 2 1900-1980 |
Historical Group 3 >1980 |
|
| Total Included Publications | 6 | 10 | 4 |
| Total Included Studies | 10 | 23 | 4 |
| Total Male Sacra Observed | 790 | 2,883 | 319 |
| Total Males Cases of SSBO Identified | 98 | 738 | 46 |
| Total Frequency | 12.40% | 25.59% | 14.42% |
| Total Increase/Decrease | N/A | +13.19% | -11.17% |
| Chi-Squared Value | N/A | HG1-HG2 40.618 |
HG2-HG3 12.209 |
| P-Value | N/A | <0.0001% | <0.0005% |
| Total Female Sacra Observed | 720 | 2,830 | 268 |
| Total Female Cases of SSBO Identified | 69 | 458 | 74 |
| Total Frequency | 9.58% | 16.18% | 27.61% |
| Total Increase/Decrease | N/A | 6.60% | +11.43% |
| Chi-Squared Value | N/A | HG1-HG2 14.737 |
HG2-HG3 14.105 |
| p-Value | N/A | <0.0001 | <0.0002 |
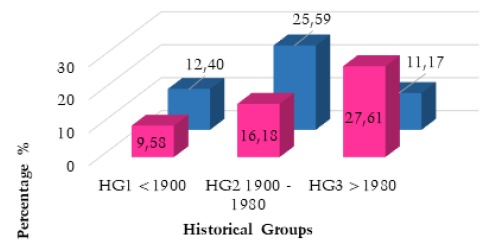
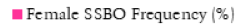
Fig. 7. Male and female frequency by historical group. Data derived from numerical analysis of included literature and frequency calculations outlined in Table 8.
Discussion
Almost all results pertaining to the overall frequency of SSBO demonstrated a substantial, statistically significant increase after 1900. These results provide compelling confirmation for the hypothesised increase in the frequency of SSBO and its correlation with the relaxation of natural selection around 1900. The importance of these results for the determination of current evolutionary change can be conceptualised by outlining the frequency for each historical group. HG1, (2,500BCE to 1900CE), had a total frequency of 13.36%, compared to HG2, (1900 to 1980) at 18.61%, which demonstrates a clear increase of 5.25% over a small 80-year period. HG3, (>1980) produced a frequency of 23.59%, and an increase of 4.98% from HG2, despite representing a smaller sample size and shorter period of history (40 years). When compared to HG1 (<1900), HG3 demonstrated an even more significant result, of a 10.23% increase in the frequency of SSBO between 1900CE and the present. When converted to increase per decade, these figures: 1.31% and 2.49%, demonstrate an accelerating non-linear increase in the frequency of SSBO in the modern era (Saniotis and Henneberg 2011).
The calculation of male and female prevalence for HG1 and HG2 also produced statistically significant results in support of an increase of SSBO after 1900. These results demonstrated higher percentages of increase than the total frequency calculation for these historical groups. Despite the sample sizes of each sex (3,992 males and 3,818 females) being similar, male prevalence was much higher and demonstrated a 6.59% greater increase than among females (13.19% male to 6.60% female) between HG1 and HG2. This could potentially represent a sex based evolutionary trend that has yet to be fully investigated, as male frequency was consistently higher with the exception of the decrease observed for males in HG3. This 11.17% decrease observed for males after 1980 is the only decrease noted across the entire analysis and is accompanied by a substantial 11.43% increase for females in this group. These calculations for >1980 are based on just four publications, with small numbers of sacra, and thus, may reflect regional differences rather than temporal trends. It is important to note that additional data are needed for HG3 to ensure that these relative increases/decreases can be substantiated.
While this research does represent the largest assessment of SSBO in the literature (16,167 sacra), the scarcity of relevant literature and the acknowledged limitations of these studies suggest that these results represent only a fraction of potentially recoverable data. The increase in the frequency of SSBO is relatively modest compared to the median artery, which shows an increase of 20% over the same 120-year period, about double that of the increase in SSBO (Lucas T et al. 2020). Similarly, tarsal coalitions experienced an increase of 12% over a 50-year period, double the reported frequency demonstrated for SSBO for this period (Ruhli et al. 2003). These modest increases in frequency recorded for SSBO may be the product of the lack of data and academic consensus. It may be possible that with the inclusion of additional, larger, and targeted datasets, rates of SSBO frequency may increase again, to parallel those observed in these other anatomical structures.
This research holds important implications for the general application of both biological anthropology and bioarchaeology. The recognition of implications of natural selection on widescale secular change can improve the accuracy of differential diagnosis in skeletal remains. Awareness of this increase in skeletal variation, its frequency, and patterns of presentation, can improve future bioarchaeological interpretations of trauma, pathology, and health status. The potential for this phenomenon to produce previously unobserved skeletal changes must also be recognised and attempts to identify pathological associations with new forms of variation must be addressed. By acknowledging that the human skeleton is changing, collaboration with the medical community and the use of clinical methodology, can strengthen the capacity of bioarchaeology to provide insight into global future health outcomes as they relate to secular changes.
The incorporation of clinical parameters, statistical calculations, and bias assessments into this bioarchaeological assessment of SSBO frequency has provided a unique opportunity to design a systematic methodology which can be applied to a range of skeletal and anatomical variations. This framework has allowed for the traditionally small scale and localised anthropological datasets to be amalgamated into a broad temporal and geographic ‘map’ of SSBO frequency, emphasising overarching patterns not identifiable in smaller studies. This method allows for small datasets to be incorporated with a high degree of accuracy and can facilitate a continuous addition of new data. Potentially, this could produce an ever increasing ‘map’ of SSBO frequency, where the addition of datasets from a range of researchers would allow the eventual creation of a truly global representation of SSBO frequency and its secular trajectory. This method could then be expanded to include additional anatomical variations, from independent or future researchers, that would also lead to the creation of global ‘maps’ of diverse conditions frequencies.
Future bioarchaeological assessments of skeletal variation should be reconceptualised, with the traditional focus on individual and localised assessments of change replaced with wide reaching systematic evaluations of broad scale frequency. Clear patterns of secular change could be reliably assessed on a global scale, and these trends systematically compared. The potential for this style of analysis to identify trends that have explicit implications for public health and medicine, can be demonstrated through the comparison of SSBO and SBC frequency. The 4.98% increase in SSBO frequency observed after 1980 demonstrates a sustained increase of this condition and conforms with Solomon et al. (2009) and Lee et al. (2011) results on the confirmation of this microevolutionary increase and secular trend, despite the introduction of folate supplementation in 1980. This is in direct opposition to expected clinical outcomes for SSBO after folate supplementation introduction, which has resulted in a sharp decline of NTD related births worldwide after 1980, (Fig. 8) (Atta et al. 2016). This would suggest that SSBO potentially does not follow the same embryonic and etiological trajectory as SBC and has a separate cause altogether. While these results do not confirm or identify the underlying cause of SSBO, the large scale and systematic nature of this assessment, provides the foundation to test such hypotheses further.
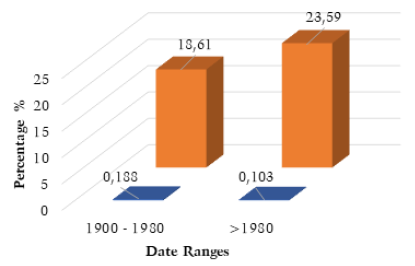

Fig. 8. Comparison between SBC and SSBO frequency by date. Resulting frequency data from literature analysis compared with reliably reported SBC prevalence.
While the results of this literature analysis were limited by the data available in the current literature, the primary objective of this analysis was achieved. The framework that this analysis has provided will facilitate the inclusion of additional SSBO data which will expand our understanding of this little-known condition and provide a uniform structure to ensure the replicability of all future research. In combination with the establishment of the most reliable frequency calculation to date, this framework will also enable the investigation of untested aspects of this condition, such as underlying etiology and additional pathological associations. Other anatomical variations, which have yet to be systematically evaluated, could also be incorporated into this framework, to establish a broader understanding of the trajectory and implications of secular evolutionary change in modern human populations.
Acknowledgments
We would like to Acknowledge Dr Teghan Lucas who initiated this project and Dr Jaliya Kumaratilake and Angela Gurr for assistance with specimen organisation. This project would not have been possible without them.
Due to the nature of this project no funding was required or requested.
Authors’ contribution
Both authors formulated the hypothesis. ERK collected data and drafted the text. MH helped with the analysis and edited text.
Conflict of interests
The Authors have no competing interests concerning this review.
Registration and Protocol
This review has not been registered as it is bioarchaeological.
The Protocol used for this review can be accessed in the appendix.
No amendments have been made due to registration or protocol.
Data Availability
PRISMA Flow Chart template 2020: http://www.prisma-statement.org/
Data for each individual study included in this analysis is available in the appendix.
References
Abera Z, Girma A, Bekele A, Oumer M. 2021. Assessment of morphological and morphometrical variations of sacral hiatus in dry human sacra in Ethiopia. Local Reg Anesth 14:25–32.
Abstracts from the Scandinavian society of anaesthesiologists, 30th congress, 10–13th June 2009, Odense, Denmark. Acta Anaesthesiol Scand Suppl 119:1–80.
Agostini S, Magrini SM, Simoncini R, Biti G, Villari N, Giannardi G. 1991. Association between testicular cancer and spina bifida occulta. Acta Oncol 30(5):579–81.
Albrecht TL, Scutter SD, Henneberg M. 2007. Radiographic method to assess the prevalence of Sacral Spina Bifida Occulta. Clin Anat 20:170–4.
Al-Dahhan MH, Mnaather AA, Munshid BA. 2020. Evaluation of spina bifida occulta in young paitents with low back pain. Eur J Mol Clin Med 7(10):4416–23.
Ali S, Azeemi AA, Shoukat S. 2014. The prevalence of spina bifida occulta in a Pakistani population: a study of dry human sacra. Anaesth pain Intensive Care 18(2):157–60.
Alles AJ, Sulik KK. 1990. Retinoic acid-induced spina bifida: evidence for a pathogenetic mechanism. Dev 108:73–81.
Altman NR, Altman DH. 1987. Magnetic resonance imaging of spinal dysraphism. Am J Neuroradiol 8(3):533–8.
Anderson FM. 1975. Occult spinal dysraphism: a series of 73 cases. Pediatr 55(6):826–835.
Aoki Y, Takahashi H, Nakajima A, Kutoba G, Watanabe A, Nakajima T, Eguchi Y, Orita S, Fukuchi H, Yanagawa N, Nakagawa K, Ohtori S. 2010. Prevalence of lumbar spondylolysis and spondylolisthesis in patients with degenerative spinal disease. Sci Rep 10(1):6739.
Armstrong S, Cloutier L, Arredondo C, Roksandic M, Matheson C. 2013. Spina bifida in a pre-Columbian Cuban population: a paleoepidemiological study of genetic and dietary risk factors. Int J Palepathol 3:19–29.
Asakura Y, Kandatsu N, Hashimoto A, Kamiya M, Akashi M, Toru K. 2009. Ultrasound-guided neuroaxial anesthesia: accurate diagnosis of spina bifida occulta by ultrasonography. J Anesth 23(2):312–13.
Asghar A, Naaz S. 2013. The volume of the caudal space and sacral canal in human sacrum. J Clin Diagn Res 7(12):2659–60.
Atta CAM, Fiest KM, Frolkis AD, Jette N, Pringsheim T, St Germain-Smith C, Rajapakse T, Kaplan GG, Metcalfe A. 2016. Global birth prevalence of spina bifida by folic acid fortification status. A systematic review and meta-analysis. Am J Pub Health 106:24–34.
Au KS, Ashley-Koch A, Northrup H. 2010. Epidemiologic and genetic aspects of spina bifida and other neural tube defects. Dev Disabil Res Rev 16:6–15.
Ausili E, Maresca G, Massimi L, Morgante L, Romagnoli C, Rendeli C. 2017. Occult spinal dysraphisms in newborns with skin markers: role of ultrasonography and magnetic resonance imaging. Childs Nerv Syst 34:285–91.
Ausili E, Maresca G, Massimi L, Morgante L, Romagnoli L, Rendeli C. 2018. Occult spinal dysraphism in newborns with skin markers: role of ultrasonography and magnetic resonance imaging. Childs Nerv Syst 34:285–91.
Avrahami E, Frishman E, Fridman Z, Azor M. 1994. Spina bifida occulta of S1 is not an innocent finding. Spine 19:12–15.
Bademci G, Saygun M, Batay F, Cakmak A, Basar H, Anbarci H. 2006. Prevalence of primary tethered cord syndrome associated with occult spinal dysraphism in primary school children in Turkey. Pediatr Neurosurg 42:4–13.
Bajpai M, Bhatnagar V, Mitra DK, Rohatgi M, Upadhyaya P. 1989. Spina bifida occulta: radiographic and operative correlation. Indian J Pediatr 56(4):513–17.
Balduzzi S, Rucker G, Schwarzer G. 2019. How to perform a meta-analysis with R: a practical tutorial. Evidence based Mental Health 22:153–60.
Banno T, Ohishi T, Suzuki D, Honda Y, Kobayashi S, Matsuyama Y. 2012. Traumatic sacral pseudomeningocele with spina bifida occulta. J Neurosurg Spine 16(1):78–81.
Barf HA, Verhoef M, Jennekens-Schinkel A, Post MWM, Gooskens RHJM, Prevo AJH. 2003. Cognitive status of young adults with spina bifida. Dev Med Child Neurol 45:813–20.
Barkely AS, Susarla SM, Lee A. 2019. Frontotemporal dermal sinus tract with two connected intradiploic dermoid cysts: a rare case and review of the literature. World Neurosurg 127:350–53.
Barnet J. 1913. A study of merorachischisis (spina bifida occulta). Am J Dis Child 5(4):287–296.
Barson AJ. 1970. Spina bifida: the significance of the level and extent of the defect to morphogenesis. Dev Med Child Neurol 12(2):129–44.
Behrooz A, Gorjizadeh MH. 2007. Prevalence and correlates of neural tube defects in a retrospective analysis of south-western Iran. Sultan Qaboos Uni Med J 7(1):31–34.
Bennett KA. 1972. Lumbo-sacral malformations and spina bifida occult in a group of proto-historic Modoc Indians. Am J Phys Anthrop 36(3):435–39.
Ben-Sira L, Garel C, Malinger G, Constantini S. 2013. Prenatal diagnosis of spinal dysraphism. Childs Nerv Syst 29(9):1541–2.
Berbrayer D. 1991. Tethered cord syndrome complicating spina bifida occulta. A case report. Am J Phys Med Rehabil. 70(4): 213–4.
Berry AC. 1975. Factors affecting the incidence of non-metrical skeletal variants. J Anat 120(3):519–35.
Bessis D. 2020. Cutaneous signs of occult cranial and spinal dysraphism. Ann Dermatol Venereol 147(8–9):504–19.
Bhalla A, Bono LM. 2019. Isthamic lumbar spondylolisthesis. Neurosurg Clin N Am 30(3):283–90.
Bhide P, Sagoo GS, Moorthie S, Burton H, Kar A. 2015. Neural tube dysraphism: a review of cutaneous markers and imaging. Peditar Dermtol 32(2):161–70.
Bilton MJ. 2003. Ethics: ‘life before birth’ and moral complexity in maternal-fetal surgery for spina bifida. Clin Perinatol 30(3):449–64.
Blom HJ, Shaw GM, Heijer MD, Finnell RH. 2006. Neural tube defects and folate: case far from closed. Nat Rev Neurosci 7(9):724–31.
Boano R, Fulcheri E, Martina MC, Ferraris A, Grilletto R, Cremo R, Cesarani F, Gandini G, Massa ER. 2009. Neural tube defect in a 4000-year-old Egyptian infant mummy: a case of meningocele from the Museum of anthropology and ethnography of Turan (Italy). Eur J Pediatr Neurol 13:481–7.
Boone D, Parsons D, Lachmann SM, Sherwood T. 1985. Spina bifida occulta: lesion or anomaly? Clin Radiol 36(2):159–61.
Bourke JB. 1971. The paleopathology of the vertebral column in ancient Egypt and Nubia. Med Hist 15(4):363–75.
Bowman RM, Boshnjaku V, McLone DG. 2009. The changing incidence of myelomeningocele and its impact on pediatric neurosurgery: a review from the Children’s Memorial Hospital. Childs Nerv Syst 25(7):801–6.
Boyuat A, Yazar T, Ekmekci P, Gurgey E. 2000. Lumbosacral vascular malformations. A hallmark for occult spinal dysraphism. Dermatol 201:374–76.
Bozkurt G, Sackesen C, Ciuelek E, Kalayci O, Akalan N, Cataltepe O. 2010. Latex sensitization and allergy in children with spina bifida in Turkey. Childs Nerv Syst 26(12):1735–42.
Bradtmiller B. 1984. Congenital anomalies of the lower spine in two Anikara skeletal series. Plains Anthropol 29(106):327–33.
Brand MC. 2006. Examining the newborn with open spinal dysraphism. Adv neonatal care 6(4):181–96.
Brasili P, Facchini F, Scarani P, Mazzucato L. 1997. Reconstruction of the health status in a past human population: the Iron Age Necropolis of Monte Bibele. (Bologna Italy). Anthrop Anz 55(3/4):247–64.
Brei TJ, Walker WO Jr. 2018. Perspectives on surgical care and outcomes in spina bifida. Pediatr 142(3):e20181958.
Bruzek AK, Starr J, Garton HJL, Muraszko KM, Maher CO, Strahle JM. 2019. Syringomyelia in children with closed spinal dysraphism: long term outcomes after surgical intervention. J Neurosurg Pediatr 13:1–7.
Buta JL. 1975. Spina bifida occulta and spina bifida cystica and related manifestations, (a review article). Mich Med 74(24):451–3.
Buwembo W, Obore AP, Ziraba S, Kange M, Munabi IG, Okori H, Namusoke F, Mwaka E, Luboga SA. 2016. Occurrence of spina bifida in the Makerere University Galloway Collection: an osteological anatomical study. Anat J Afr 5(2):952–6.
Cai C, Shen C, Yang W, Zhang Q, Hu X. 2008. Intraspinal neurenteric cysts in children. Can J Neurol Sci 35(5):609–15.
Cairns RB, Gariepy JL. 1990. Development, microevolution, and social behaviour. Psychol Rev 97:49–65.
Cakiroglu B, Arda E, Tas T, Senturk AB. 2018. Alarm therapy and decompression in the treatment of patients with nocturnal enuresis. Afr J Paediatr Surg 15(3–4):131–4.
Cakiroglu B, Tas T, Eyyupoglu SE, Hazar AI, Can Balci MB, Nas Y, Yilmazer F, Aksoy SH. 2014. The adverse influence of spina bifida occulta on the medical treatment outcome of primary monosymptomatic nocturnal enuresis. Arch Ital Urol Androl 86(4):270–73.
Capitanucci ML, Iancobelli BD, Silveri M, Mosiello G, De Gennaro M.1996. Long-term urological follow-up of occult spinal dysraphism in children. Eur J Pediatr Surg 6(1):25–26.
Carson JA, Barnes PD, Turnell WP, Smith EI, Jolley SG. 1984. Imperforate anus: the neurologic implication of sacral abnormalities. J Pediatr Surg 19(6):383–42.
Carstairs V, Cole S. 1984. Spina bifida and anencephaly in Scotland. Brit Med J 289(6453):1182–4.
Carter CO, Evans K. 1973. Spina bifida and anencephaly in greater London. J Med Genet 10:209–35.
Carter CO, Evans KA, Till K. 1976. Spinal dysraphism: genetic relation to neural tube malformations. J Med Genet 13:343–50.
Carter GT. 2014. Spinal cord injury rehabilitation. Phys Med Rehabil Clin N Am 25(3):13–14.
Cassar P. 1983. The birth of monsters in the Maltese Islands in the 17th and 18th centuries. Medi-scope 1:6–9.
Castilla EE, Orioli IM, Lopez-Camelo JS, Dutra DG, Nazer-Herrera J. 2003. Preliminary data on changes in neural tube defect prevalence rates after folic acid fortification in South America. Am J Med Genet 123(2):123–8.
Castro de la Mata R, Bonavia D. 1980. Lumbosacral malformations and spina bifida in a Peruvian preceramic child. Current Anthropol 21(4):515–6.
Chan A, Robertson EF, Haan EA, Keane RJ, Ranieri E, Carney A. 1993. Prevalence of neural tube defects in South Australia, 1966–1991: effectiveness and impact of prenatal diagnosis. Adel Brit Med J 307(6906):703.
Chan AC, Essen P, Scott H, Haan EA, Sage L, Scott J, Gill TK, Nguyen AMT. 2008. Folate awareness and the prevalence of neural tube defects in South Australia, 1966–2007. Med J Aus 189(10):566–70.
Chauhan N, Agashe A, Gopal S, Paranjpe S. 2015. Uterine procidentia in a 20-year-old unmarried nulliparous woman: a case report. J Evol Med Dent Sci 4(97):e16290.
Chen G, Pei LJ, Huang J, Song XM, Lin LM, Gu X, Wu JX, Wang F, Wu JI, Chen JP, Liu JF, Xin RL, Zhang T, Zheng XY. 2009 Unusual patterns of neural tube defects in a high risk region of northern China. Biomed Enviro Sci 22:340–4.
Cheung EV, Herman MJ, Cavalier R, Pizzutillo PD. 2006. Spondylolysis and spondylolisthesis in children and adolescents: II. Surgical management. J Am Acad Orthop Surg 14(8):488–98.
Chi BH, Moon YT, Myung SC, Kim KD, Kim K, Chang IH, Kim JW. 2017. The prevalence and clinical features of spinal dysraphism in children with hypoplasia. Eur Urol Suppl 16(3):e1048.
Chiaretti A, Rendeli C, Antonelli A, Barone G, Focarelli B, Tabacco F, Massimi L, Ausili E. 2008. GDNF plasma levels in spina bifida: correlation with severity of spinal damage and motor function. J Neurotrauma 25(12):1477–81.
Cho DY, Leipold HW. 1977. Spina bifida and spinal dysraphism in calves. Zentralbl Veterinar Med A 24(8):680–95.
Choi SJ, Yoon HM, Hwang JS, Suh, CH, Jung AY, Cho YA, Lee JS. 2020. Incidence of occult spinal dysraphism among infants with cutaneous stigmata and proportion managed with neurosurgery: a systematic review and meta-analysis. JAMA Newt Open 3(7):e207221.
Cilione M, Gazzaniga V. 2021. Did Hippocrates know spina bifida? Spine J 21(5):841.
Cockroft DL. 1991. Vitamin deficiencies and neural tube defects: human and animal studies. Hum Reproduc 6(1):148–57.
Copp AJ, Greene NDE. 2010. Genetics and development of neural tube defects. J Pathol 220:217–30.
Copp AJ, Greene NDE. 2013. Neural tube defects – disorders of neurulation and related embryonic processes. Dev Biol 2:213–27.
Cornette L, Verpoorten C, Lagae L, Plets C, Van Calenbergh F, Casaer P. 1998. Closed spinal dysraphism: a review on diagnosis and treatment in infancy. Eur J Pediatr Neurol 2(4):179–85.
Cotter AM, Daly SF. 2005. Neural tube defects: is a decreasing prevalence associated with a decrease in severity? Eur J Obstet Gynecol Repor Bio 119:161–3.
Cragg JJ, Warner FM, Shupler MS, Jutzeler CR, Cashman N, Whitehurst DGT, Kramer JK. 2018. Prevalence of chronic pain among individuals with neurological conditions. Health Rep 29(3):11–6.
Cross JF. 1988. The skeletal biology of two late medieval eastern Scottish populations recovered from Carmlite Friaries in Aberdeen and Perth. Proquest Dissertation Publishing.
Crowe CA, Heuther CA, Oppenheimer SG, Barth LD, Jeffery E, Reinhart S. 1985. The epidemiology of spina bifida in south-western Ohio – 1970–1979. Dev Med Child Neurol 27:176–82.
Curcio MR, Ferrantis S, Lotti F, Grosso S. 2021. Coffin-Saris syndrome and epilepsy. Neurol Sci 42(2):727–9.
Czeizel AE, Dudas I, Vereczkey A, Banhidy F. 2013. Folate deficiency and folic acid supplementation: the prevention of neural tube defects and congenital heart defects. Nutrients 5(11):4760–75.
Damkier P, Bronniche LMS, Korch-Frandsen JFB, Broe A. 2019. In utero exposure to anti-biotics and risk of congenital malformations: a population-based study. Am J Obstet Gynecol 221(6):1–5.
De Anquin CE. 1959. Spina bifida occulta with engagement of the fifth lumbar spinous process. A cause of lower back pain and sciatica. J Bone Joint Surg 41(3):486–90.
De Gennaro M, Rivosecchi M, Lucchetti MC, Silveri M, Fariello G, Schingo P. 1994. The incidence of occult spinal dysraphism, and the onset of neurovesical dysfunction in children with anorectal anomalies. Eur J Pediatr Surg 4(1):12–4.
Deeg KH, Lode HM, Gassner I. 2008. Spinal sonography in newborns and infants – part II: spinal dysraphism and tethered cord. Ultraschall Med 29(1):77–88.
Degenhardt P, Golla S, Wahn F, Niggemann B. 2001. Latex allergy in pediatric surgery is dependent on repeated operations in the first year of life. J Pediatr Surg 36(10):1535–9.
Detrait ER, George TM, Etchevers HC, Gilbert JR, Vekemans M, Speer MC. 2005. Human neural tube defects: developmental biology, epidemiology, and genetics. Neurotoxicol teratol 27:515–24.
Devor EJ, Cordell LS. 1981. Neural tube defects in a prehistoric south-western Indian population. Ann Hum Bio 8(1):65–75.
Dhaulakhandi DB, Rohilla S, Rattan KN. 2010. Neural tube defects: review of experimental evidence on stem cell therapy and newer treatment options. Fetal Diagn Ther 28(2):72–8.
Dicianno BE, Fairman AD, Juengst SB, Braun PG, Zabel TA. 2010. Using the spina bifida life course model in clinical practice: an interdisciplinary approach. Pediatr Clin N Am 57(4):945–57.
Dickel DN, Doran GH. 1989. Severe neural tube defect syndrome from the early archaic of Florida. Am J Phys Anthrop 80:325–34.
Diel J, Ortiz, O, Losada RA, Price DB, Hayt MW, Katz DS. 2001. The sacrum: pathologic spectrum, multi-modality imaging, and susceptibility approach. Radiographics 21(1):83–104.
Diyora B, Bhende B, Kukreja S. 2018. Giant craniospinal intermedullary neurenteric cyst in infant-case report and review of literature. World Neurosurg 118:126–31.
Dodd K. 1984. Where should spina bifida children go to school? Z Kinderchir 39(2):129–31.
Dossche L, Walle JV, Van Herzeeke C. 2016. The pathophysiology of monosymptomatic nocturnal enuresis with special emphasis on the circadian rhythm of renal physiology. Eur J Pediatr 175(6):747–54.
Dritsoula AK, Thevasagayam MS. 2015. Congenital aplasia/hypoplasia of the Epiglottis – a case report and review of the literature. Int J Pediatr Otorhinolaryngol 79(10):1609–12.
Drolet BA, Boudreau C. 2004. When good is not enough. The predictive value of cutaneous lesions of the lumbosacral region for occult spinal dysraphism. Arch Dermatol 140(9):1153–5.
Drolet BA, Chamlin SL, Garzon MC, Adams D, Baselga E, Haggstrom AN, Holland KE, Horii KA, Juern A, Lucky AW, Mancini AJ, McCuaig C, Metry DW, Morel KD, Newell BD, Nopper AJ, Powell J, Frieden IJ. 2010. Prospective study of spinal anomalies in children with infantile hemangiomas of the lumbosacral skin. J Pediatr 157(15):789–94.
Dudar JC. 2010. Qualitative and quantitative diagnosis of lethal cranial neural tube defects from the fetal and neonatal human skeleton, with a case study involving taphonomically altered remains. J Forensic Sci 55(4):877–83.
Egloff A, Bulas D. 2015. Magnetic resonance imaging evaluation of fetal neural tube defects. Semin Ultrasound, CT and MRI 36(6):487–500.
Ehara H, Ohno K, Ohtani K, Kueda T, Takeshita K. 1998. Epidemiology of spina bifida in Tottori Prefecture, Japan, 1976–1995. Pediatr Neurol 19(3):199–203.
Ekwochi U, Asinobi IN, Osuorah DCI, Ndu IK, Ifediora C, Amadi OT, Sunday G. 2018. Pattern of congenital anomalies in newborns delivered in a tertiary healthcare facility in the South-East Nigeria. J Trop Pediatr 64(4):304–11.
El-Awad ME, Sivasankaran S. 1992. Neural tube defects in southwestern region of Saudi Arabia. Ann Scudi Med 12(5):449–52.
El-Din A, El Banna R. 2006. Congenital anomalies of the vertebral column: a case study on ancient and modern Egypt. Int J Osteoarchaeol 16:200–7.
Eldridge C, Bandlamuri S, Andrews JG, Galindo MK, Contreras D, Flood TJ, Rice S. 2018. Post folate spina bifida lesion level change. Birth Defects Res 110(11):949–55.
Elshani B, Lenjani B. 2013. Comparison of hydrocephalus appearance at spinal-dysraphia. Med Arch 67(3):188–91.
Elwood JH. 1973. Epidemics of anencephaly and spina bifida in Ireland since 1900. Int J Epidemiol 2(2):171–5.
Estebaranz-Sanchez F, Martinez LM, Alrousan M, Chamel B, Molist M, Coqueugniot E, Perez-Perez A. 2018. Spinal dysraphism at the Syrian neolithic site of Dia’de El-Mughara. Arc Anthrop Sci 10:1375–87.
Eubanks JD, Cheruvu VK. 2009. Prevalence of sacral spina bifida occulta and its relationship to age, sex, race, and the sacral table angle. Spine 34:1539–43.
Falci SP, Indeck C, Lammertse DP. 2009. Posttraumatic spinal cord tethering and syringomyelia: surgical treatment and long-term outcome. J Neurosurg Spine 11(4):445–60.
Fawcitt J. 1959. Some radiological aspects of congenital anomalies of the spine in childhood and infancy. J Royal Soc Med 52(5):331–3.
Feldkamp M, Friedrichs M, Carey JC. 2002. Decreasing prevalence of neural tube defects in Utah, 1985–2000. Teratol 66:23–28.
Ferembach D. 1963. Frequency of spina bifida occulta in prehistoric human skeletons. Nat 199:100–2.
Feuchtbaum LB, Currier RJ, Riggle S, Roberson M, Lorey FW, Cunningham C. 1999. Neural tube defect prevalence in California (1990–1994): eliciting patterns by type of defect and maternal race/ethnicity. Genet test 3(3):265–73.
Fidan F, Cay N, Asilturk M, Veizi E. 2021.The incidence of congenital lumbosacral malformations in young male Turkish military school candidates’ population. J Orthop Sci 1–5.
Fidas A, MacDonald HL, Elton RA, McInnes A, Brown A, Chrisholm GD. 1989. Neurophysiological measurements in patients with genuine stress incontinence of urine and the relation of neurogenic defects to the presence of spina bifida occulta. Brit Med J 289:357–60.
Fidas A, MacDonald HL, Elton RA, Wild SR, Chrisholm GR, Scott R. 1987. Prevalence and patterns of spina bifida occulta in 2707 normal adults. Clin Rad 4:537–42.
Field B. 1978. Neural tube defects in New South Wales, Australia. J Med Genet 15:329–338.
Fineman RM, Jorde LB, Martin RA, Hasstedt SJ, Wung SD, Walker ML. 1982. Spinal dysgraphia as an autosomal dominant defect in four families. Am J Med Genet 12:457–64.
Fong CY, Ong FN, Ong LC, Khoo TB, Lee ML. 2020. Vitamin D deficiency and insufficiency in Malaysian children with spina bifida. Spinal Cord 58(9):1030–6.
Frey L, Hauser WA. 2003. Epidemiology of neural tube defects. Epilepsia 44(3):4–13.
Gadioli G, Scaggion C, Carrara N. 2018. Anthropological analysis and paleopathological demographic study of human skeletal remains from the late ancient Necropolis of Biverone (4th-5th century AD). San Stino Di Livenza (Venice, Italy). Anthrop Rev 81(8):66–80.
Garralda MD, Herrerin J, Vandermeersch B. 2002. Child pathology in the Medicants Necropolis of El Burgo de Osma Cathedral (Soria, Spain). Bull Mem Soc Anthrop (Paris) 14(3–4):311–25.
Ge CY, Hao DJ, Shan LQ. 2020. Rare bony diastematomyelia associated with intraspinal teratoma. World Neurosurg 133:185–8.
Gedefaw A, Teklu S, Tadesse BT. 2018. Magnitude of neural tube defects and associated risk factors at three teaching hospitals in Addis Ababa, Ethiopia. Biomed Res Int 2018:4829023–10.
George P, Maria T, Panagiotis K. 2013. Lumbosacral transitional vertebrae associated with sacral spina bifida occulta: a case report. Acta Medica (Hradec Kraloue) 56(3):126–9.
Gerszten PC, Gerszten E, Allison MJ. 2001. Diseases of the spine in South American mummies. Neurosurg 48(1):208–13.
Gibson PJ, Britton J, Hall DM, Hill CR. 1995. Lumbosacral skin markers and identification of occult spinal dysraphism in neonates. Acta Pediatr 84(2):208–9.
Golalipour MJ, Ahmadpour-Kacho M, Vakili MA. 2005. Congenital malformations at a referral hospital in Gorgan, Islamic Republic of Iran. La Revue Mediterr Orient 11:707–15.
Goldstein MS, Arensburg B, Nathan H. 1976. Pathology of Bedouin skeletal remains from two sites in Israel. Am J Phys Anthrop 45(3):621–39.
Goldstein MS. 1957. Skeletal pathology of early Indians from Texas. Am J Phys Anthrop 15(3):299–311.
Greene VW. 2001. Personal hygiene and life expectancy improvements since 1850: historic and epidemiological associations. Am J Infect Control 29:203–6.
Groza VM, Simalcsik A, Bejenaru L. 2012. Frequency of spina bifida occulta and other occult spinal dysraphism’s in the medieval population of Isas city: skeleton palaeopathology in the necropolis discovered in the eastern part of the Princely Court, 17th century. Biol Anim 58:195–204.
Groza VM, Simalcsik A, Bejenaru L. 2013. Spina bifida occulta in medieval and post-medieval skeletons from Iasi City, in north-east Romania. Biol Anim 59:101–4.
Gu X, Lin L, Zheng X, ZHANG t, Song X, Wang J, Li X, Li P, Chen G, Wu J, Wu L, Liu J. 2007. High prevalence of NTD’s in Shanxi Province: a combined epidemiological approach. Birth Defects Res 79:702–7.
Guggisberg D, Hadj-Rabia S, Viney C, Bodemer C, Brunelle F, Zerah M, Pierre-Kahn A, de Prost Y, Hamel-Teillac D. 2004. Skin markers of occult spinal dysraphism in children: a review of 54 cases. Arch Dermatol 140(9):1109–15.
Gupta SK, Gupta RC, Seth AK, Chattucridi CS. 1995. Increased incidence of spina bifida occulta in fluorosis prone areas. Acta Pediatr Jpn 37(4):503–6.
Gupta SK, Khosla VK, Sharma BS, Mathuriya SN, Pathnak A, Tewari MK. 1999. Tethered cord syndrome in adults. Surg Neurol 52(4):362–70.
Hall JG, Friedman JM, Kenna BA, Popkin J, Jawanda M, Arnold W. 1988. Clinical, genetic, and epidemiological factors in neural tube defects. Am J Hum Genet 43:827–37.
Hamill N, Grant JA, Myers SA. 2008. Congenital dermal sinus. J Ultrasound Med 27(5):799–802.
Harada A, Nishiyama K, Yoshimura J, Sano M, Fujii Y. 2014. Intraspinal lesions associated with sacrococcygeal dimples. J Neurosurg Pediatr 14(1):81–6.
Henneberg RJ, Henneberg M. 1999. Variation in the closure of the sacral canal in the skeletal sample from Pompeii, Italy, 79AD. Perspect Hum Bio 4:177–188.
Hettige S, Smart C, Bridges LR, Martin AJ. 2012. Paciniolipoma in congenital spinal dysraphism. J Neurosurg 9(3):280–2.
Hewitt D. 1963. Geographic variations in the mortality attributed to spina bifida and other congenital malformations. Brit J Prev Soc Med 17:13–23.
Higgins JPT, Thomson SG, Deeks JJ, Altman DG. 2003. Measuring inconsistency in meta-analyses. Brit Med J 327:557–60.
Hirsh JF, Pierre-Kahn A. 1988. Lumbo-sacral lipomas with spina bifida. Childs Nerv Syst 4(6):354–60.
Hobbs MST. 1969. Risk of anencephaly in the migrant and non-migrant women in the Oxford area. Brit J Prev Soc Med 23:174–8.
Hoffman ED. 1965. The problems of spina bifida and cranium bifidum. A survey of contemporary ideas. Clin Pediatr 4(12):709–16.
Hofmann MI, Boni T, Alt KW, Woitek U, Ruhli FJ. 2008. Paleopathologies of the vertebral column in medieval skeletons. Anthrop Anz 66(1):1–17.
Hol FA, Geurds MPA, Chatkupt S, Shugart YY, Balling R, Schrander-Stumper CTRM, Johnson WG, Hamel BCJ, Mariman ECM. 1996. PAX genes and human neural tube defects: an amino acid substitution in PAX1 in a patient with spina bifida. J Med Genet 33:655–60.
Hollander WF. 1976. Genetic spina bifida occulta in the mouse. Am J Anat 146(2):173–9.
Holmes LC, Li V. 2019. Occult spinal dysraphism. Pediatr Rev 40(12):650–52.
Horn SM Moses M, Vasquez-Montes D, Hockley A, Poorman G, Bortz C, Segreto FA, Brown AE, Pierce KE, Alas H, Ihejirika YU, Moon J, Varlotta CG, Vira S, Diebo BG, Ramos RG, Lafage R, Lafage V, Sciubba DM, Raad M, Nikas D, Passias PG. 2020. Tethered cord syndrome in the United States: cluster analysis of presenting anomalies and associated. Bull Hosp J Dis 78(3):157–62.
Humphreys RP, Hendrick EB, Hoffman HJ. 1983. Diastematomyelia. Clin Neurosurg 30:436–456.
Hussien FH, El-Din AM, Kandeet W, El Banna R. 2009. Spinal pathological findings in ancient Egyptians of the Greco-Roman period living in Bahriyah Oasis. Int J Osteoarchaeol 19:613–27.
Iam S, Barry J, Dauser RC. 2014. Dermal sinus tract: clinical presentation and imaging findings. Pediatr Neurol 51(5):747–8.
Ingram CJE, Mulcare CA, Itan Y, Thomas MG, Swallow DM. 2009. Lactose digestion and the evolutionary genetics of lactase persistence. Hum Genet 124:579–91.
Iskander BJ, Oakes WJ, McLaughlin C, Osumi AK, Tien RD. 1994. Terminal syringohydromyelia and occult spinal dysraphism. J Neurosurg 81(4):513–9.
Jai S, Wei X, Ma L, Wang Y, Gu H, Liu D, Ma W, Yuan Z. 2019. Maternal, paternal, and neonatal risk factors for neural tube defects: a systematic review and meta-analysis. Int J Dev Neurosci 78:227–35.
James CC. Spina bifida occulta. 1979. S Afr Med J 55(26):1056.
James CCM. 1959. The need for orthopedic treatment of infants with delayed physical development. Pract 182(1091):598–602.
James WH. 1979. The sex ratio in spina bifida. J Med Genet 16(5):384–8.
Jankauskas R. 2001. Variations and anomalies of the vertebra column in Lithuanian palaeoosteological samples. Anthropol 39:33–8.
Jensson O, Arnason A, Gunnarsdottir H, Petursdottir I, Fossdal R, Hreidarsson S. 1988. A family showing apparent X linked inheritance of both anencephaly and spina bifida. J Med Genet 25:227–9.
Johnson KC, Rouleau J. 1997. Temporal trends in Canadian birth defects, birth prevalence’s, 1979–1993. Canad J Public Health 88(3):169–76.
Jozsa L, Pap I, Budapest EF. 1992. The occurrence of spina bifida occulta in medieval and contemporaneous Hungarian populations. Anthropol Hunarica 22:51–60.
Jung SC, Kim SS, Yoon KS, Lee JS. 1999. Prevalence of congenital malformations and genetic diseases in Korea. J Human Genet 44:30–4.
Kajbafzadeh A, Espander L, Mehdizadeh M, Tajik P, Mohsemi P. 2004. Spina bifida occulta in persistent primary nocturnal enuresis. Iran J Radiol 2004:65–7.
Kallen B, Lofkvist E. 1984. Time trends of spina bifida in Sweden 1947–81. J Epidemiol Community Health 38:103–7.
Kanburoglu MK. 2016. Not just a capillary hemangioma. World J Pediatr 12(2):249.
Karim Ahmed A, Howell EP, Harward S, Sankey EW, Ehresman J, Schilling A, Wang T, Pennington Z, Gray L, Sciubba DM, Goodwin CR. 2020. Split cord malformation in adults: literature review and classification. Clin Neurol Neurosurg 193:105733.
Karlin IW. 1935. Incidence of spina bifida occulta in children with and without enuresis. Am J Dis Child 3:374–93.
Karmarkar SJ. 1997. Spina bifida clinic-organisational aspects. Indian J Pediatr 64(6):83–85.
Kato K, Fujiki K. 1996. Incidence of congenital malformations in Tokyo Metropolitan Hospitaks, 1979–1993. Brain Dev 18:230–3.
Kellock WL, Parsons PA. A comparison of the incidence of minor non-metrical cranial variants in Australian Aboriginies with those of Malaysia and Polynesia. Am J Phys Anthrop 33:235–40.
Khalatbari H, Perez FA, Lee A, Shaw DWW. 2020. Rapid magnetic resonance imaging of the spine in neonates with spinal dysraphism. World Neurosurg 144:648–59.
Kim DW, Lee SJ, Choi EJ, Lee PB, Jo YH, Nahm FS. 2014. Morphological diversities of sacral canal in children: three-dimensional computed tomography study. Korean J Pain 27:253–59.
Kim Y, Kim H, Hong JH, Lee HJ, Kim MJ, Shin DH. 2018. Lumbosacral defects in a 16th – 18th century Joseon Dynasty skeletal series from Korea. Biomed Res Int 28:1–8.
Koksel T, Revesz T, Crockard HA. 1990. Craniospinal neurenteric cyst. Brit J Neurosurg 4(5):425–8.
Koo BN, Hong JY, Song HT, Kim JM, Kil HK. 2012. Ultrasonography reveals a high prevalence of lower spinal dysraphism in children with urological anomalies. Acta Anaesthesiol Scand 56:624–8.
Kozlov N, Bhattarai B. 2019. Spina bifida occulta and surgical treatment in a Yorkshire terrier. J Small Anim Pract 60(10):636.
Kriss VM, Desai SN. 1998. Occult spinal dysraphism in neonates. Assessment of high-risk cutaneous stigmata on Sonography. Am J Radiol 171:1687–92.
Kriss VM, Kriss TC, Desai NS, Warf BC. 1995. Occult spinal dysraphism in the infant. Clin Pediatr (Phila) 34(12):650–54.
Kubauat DM, Nagar SK, Lakhani C. 2013. A study of non-fusion of laminae of the first sacral vertebrae in Western India. Int J Recent Trends Sci Tech 6:122–4.
Kucera JN, Coley I, O’Hara S, Kosnik EJ, Coley BD. 2015. The simple sacral dimple: diagnostic yield of ultrasound in neonates. Pediatr Radiol 45(2):211–6.
Kumar A, Kanojia RK, Saili A. 2014. Skin dimples. Int J Dermatol 53(7):789–97.
Kumar A, Tubbs RS. 2011. Spina bifida: a diagnostic dilemma in palaeopathology. Clin Anat 24:19–33.
Kumar J, Afsal M, Garg A. 2017. Imaging spectrum of spinal dysraphism on magnetic resonance: a pictorial review. World J Radiol 9(4):178–90.
Kumar P, Aneja S, Kumar R, Taluja V. 2005. Spina bifida occulta in functional enuresis. Indian J Pediatr 22(3):223–5.
Kumar R, Singh SN. 2003. Spinal dysraphism: trends in northern India. Pediatr Neurosurg 38:133–45.
Kuntz C 4th, Park TS. 2010. Tethered cord. Introduction. Neurosurg Focus 29(1):1.
Kurku HK. 2013. Skeletal variability in the pelvis and limb skeleton of humans: does stabalising selection limit female pelvic variation? Am J Hum Bio 25:795–802.
Lanier Jr RB. 1939. The presacral vertebrae of American white and negro males. Am J Phys Anthrop 25(3):341–20.
Lapsiwala SB, Iskander BJ. 2004. The tethered cord syndrome in adults with spina bifida occulta. Neuro Res (New York) 26(7):735–40.
Lary JM, Edmonds LD. 1996. Prevalence of spina bifida a birth – United States, 1983–1990: a comparison of two surveillance systems, MMWR Surveillance Summ 45(2):15–26.
Lassman LP, James CC. 1977. Meningocele manque. Childs Brain 3(1):1–11.
Le HK, Cardona-Grav D, Chiang G. 2019. Evaluation, and long-term management of neurogenic bladder in spinal dysraphism. Neo Rev 20(12):711–24.
Lee SM, Cheon JE, Choi YH, Kim IO, Kim WS, Cho HH, Lee JY, Wang KC. 2017. Limited dorsal myeloschisis and congenital dermal sinus: comparison of clinical and magnetic resonance imaging features. Am J Neuroradiol 38(1):176–82.
Lee YC, Solomon LB, Ruhli FJ, Schiess R, Ohrstrom L, Sullivan T, Alkadhi H, Henneberg M. 2011. Confirmation of microevolutionary increase in spina bifida occulta among Swiss birth cohorts. Eur Spine 20:776–80.
Leonard CO, Freeman JM. 1981. Spina bifida: a new disease. Pediatr 68(1):136–7.
Li X, Zhu J, Wang Y, Mu D, Dai L, Zhou G. 2013. Geographic and urban – rural disparities in the total prevalence of neural tube defects and their subtypes during 2006–2008 in China: a study using the hospital-based birth defects surveillance system. BMC Public Health 13:161–8.
Lin KL, Wang HS, Chou ML, Lui TN. 2002. Sonography for detection of spinal dermal sinus tracts. J Ultrasound Med 21(8):903–7.
Liu J, Li Z, Greene NDE, Li H, Ren A. 2017. The recurrence risk of NTD’s in a population with high prevalence of NTD’s in northern China. Oncotarget 8(42):72577–83.
Liu J, Yang GZ, Zhou JL, Cao SP, Chau DHW, Kung HF, Lin MC. 2007. Prevalence of neural tube defects in economically and socially deprived area of China. Childs Nerv Syst 23:1119–24.
Liu J, Zhang L, Li Z, Jin L, Zhang Y, Ye R, Liu J, Ren A. 2016. Prevalence and trends of neural tube defects in five countries in Shanzi Province of northern China, 2000–2004. Birth Defects Res A Clin Mol Teratol 106(4):267–74.
Lorber J, Levick K. 1967. Spina bifida cystica: incidence of spina bifida occulta in parents and controls. Arch Dis Child 42:171–173.
Lorber J, Ward AM. 1985. Spina bifida – a vanishing nightmare? Arch Dis Child 60:1086–91.
Lovett AA, Gatrell AC. 1988. The geography of spina bifida in England and Wales. Trans Institute Brit Geogr 13(3):288–302.
Lucas T, Kumaratilake J, Henneberg M. 2020. Recently increased prevalence of the human median artery of the forearm. A microevolutionary change. J of Anat 237:623–31.
Ma L, Ouyang Y, Qi Q, Hao N, Zhao D, Jiang Y, Meng H. 2018. Trisomy 22 with long spina bifida occulta: a case report. Med (Balt) 97(39):e12306.
Maat GJ, Lonnee HA, Noordhuizen HJ. 1990. Analysis of human skeletons from a Hellenistic period, buried at a ruined Bronze Age building on Failaka, Kuwait. Maison de l’Oreint 18:85–102.
MacCurdy GG. 1923. Human skeletal remains from the highlands of Peru. Am J Phys Anthrop 6(3):218–329.
MaClean MIT, MacLeod A. 1984. Seasonal variation in the frequency of anencephalous and spina bifida births in the United Kingdom. J Epidemiol Community Health 38:99–102.
MaClean MIT, MacLoud A. 1984. Seasonal variation in the frequency of anencephalous and spina bifida births in the United Kingdom. J Epidemiol Community Health 38:99–102.
Mahato NK. 2016. Implications of structural variations in the human sacrum: why is an anatomical classification crucial? Surg Radiol Anat 38:947–54.
Majumdar I, Kundu R, Das J, Mukherjee D. 2019. Dorsal dermal sinus presenting as quadriparesis. Brit Med J 12(6):e228503.
Malgosa A, Aluja MP, Isidro A. 1996. Pathological evidence in newborn children from the sixteenth century in Huelva (Spain). Intl J Osteoarchaeol 6:388–96.
Mallmann MR, Reutter H, Muller AM, Geipel A, Berg C, Gembruch U. 2017. Omphalocele-extrophy-imperforate anus-spinal defects complex: associated malformations in 12 new cases. Fetal Diagn Ther 41(1):66–70.
Malwatkar RC, Bhosale YJ. 2016. Study on morphological variability of sacral bones. J Evol Med Dent Sci 5(65):4606–9.
Manenti G, Iundusi R, Picchi E, Marsico S, D’Onofrio A, Rossi G, Tarantino U, Flores R. 2017. Anatomical variation: T1 spina bifida occulta. Radiological findings. Radiol Case Rep 12:207–9.
Marcsik A, Fothi E, Hegyi A. 2002. Paleopathological changes in the Carpathian Basin in the 10th and 11th centuries. Acta Biologica Szegediensis 46(1–2):95–99.
Marioka T, Murakami N, Shimogawa T, Mukae N, Hashiguchi K, Suzuki SO, Iihara K. 2017. Neurosurgical management and pathology of lumbosacral lipomas with tethered cord. Neuropathology 37(5):385–92.
Martinez-Cridado Y, Fernandez-Pineda I, Merchante E, Rivero-Garcia M, Bernabeu-Wittel J. 2014. Capillary malformation in the lumbosacral region as a clinical sign of occult spinal dysraphism. Int J Dermatol 53(11):538–40.
Martinez-Lage JF, Lopez-Guerrero AL, Piqueras C, Almagro MJ, Gilabert A. 2015. Intracranial haemorrhage following surgery for occult spinal dysraphism: a case-based update. Childs Nerv Syst 31(6):837–42.
Martinez-Lage JF, Villarejo Ortega FJ, Galarza M, Felipe-Murcia M, Almagro MJ. 2010. Coccygeal dermal sinus: clinical relevance and management. An Pediatr (Barc) 73(6):352–6.
Masnicova S, Benus R. 2003. Developmental anomalies in skeletal remains from the great Moravia and Middle Ages cemeteries at Devin, (Slovakia). Intl J Osteoarchaeol13:266–74.
Massimi L, Peraio S, Peppucci E, Tamburrini G, Di Rocco C. 2011. Section of the filum terminale: is it worthwhile in Chiari type 1 malformation? Neurol Sci 23(3):49–51.
Mays S. 2006. Spondylolysis, spondylolisthesis, and lumbo-sacral morphology in a medieval English skeletal population. Am J Phys Anthropol 131:352–62.
McComb JG. 2015. A practical clinical classification of spinal neural tube defects. Childs Nerv Syst 31:1641–57.
McDonnell R, Delany V, O’Mahony MT, Lynch, McKeating A, McKeating A, Monteith C, Turner MJ. 2018. An audit of neural tube defects in the republic of Ireland for 2012–2015. Ir Med J 111(3):712.
McGovern M, Mulligan S, Carney O, Wall D, Moylett E. 2013. Ultrasound investigation of sacral dimples and other stigmata of spinal dysraphism. Arch Dis Child 98(10):784–6.
McGrath M, Tayles N. 2004. Anatomical observations related to radiological findings in spina bifida -occulta of the lumbo-sacral spine. J Osteopath Med 7:70–8.
McLone DG. 1989. Spina bifida today: problems adults face. Semin Neurol 9(3):169–75.
Merbs CF. 2004. Sagittal clefting of the body and other vertebral development errors in Canadian Inuit skeletons. Am J Phys Anthropol 123:236–49.
Mete M, Umur AS, Duransoy YK, Barutcuoglu M, Umur N, Gurgen SG, Selcuki M. 2014. Congenital dermal sinus tract of the spine: experience of 16 patients. J Child Neurol 29(10):1277–82.
Miller JL, Groves ML, Baschat AA. 2019. Fetoscopic spina bifida repair. Minerva Ginecol 71(2):163–70.
Miller JR, Fraser FC, MacEwan DW. 1962. The frequency of spina bifida occulta and rib anomalies in the parents of children with spina bifida aperta and meningocoele. Am J Hum Genet 14:245–8.
Mirfazeli A, Kaviany N, Hosseinpoor K, Aryaie M, Golalipoue MJ. 2018. Birth defects in northern Iran (2008–2013). Iran J Public Health 47(3):413–7.
Mishra VV, Nanda S, Aggarwal R, Tanvir. 2016. Successful pregnancy outcome in an operated case of lipomeningomyocele: a rare case. J Clin Diagn Res 10(9):4–5.
Missmer SA, Suarez L, Felkner M, Wang E, Merill AH, Rothman KJ, Hendricks KA. 2006. Exposure to Fumonisins and the occurrence of neural tube defects along the Texas-Mexico border. Enviro Health Persp 114(2):237–41.
Mitchel LE, Duffy DL, Duffy P, Bellingham G, Martin NG. 1997. Genetic effects on variation in red-blood-cell folate in adults: implications for the familial aggregation of neural tube defects. 60:433–8.
Mitchell LE, Adzick NS, Melchionne J, Pasquariello PS, Sutton LN, Whitehead AS. 2004. Spina bifida. Lancet 364(9448):1885–95.
Miyazato M, Sagaya K, Nishijima S, Owan T, Ogawa Y. 2007. Location of spina bifida occulta and ultrasonographic bladder abnormalities predict the outcome of treatment for primary nocturnal enuresis in children. Int J Urol 14:33–8.
Mohamadzadeh N, Zirak Javanmard M, Karimipour M, Farjah G. 2021. Developmental toxicity of the neural tube induced by titanium dioxide nanoparticles in mouse embryos. Avicenna J Med Biotechnol 13(2):74–80.
Mohd-Zin SW, Maewan AI, Chaar MKA, Ahmed-Annuar A, Abdul-Aziz NM. 2017. Spina bifida: pathogenesis, mechanisms and genes in mice and humans. Scientifica (Cairo) 2017:27–9.
Molloy AM, Pangilinan F, Brody LC. 2017. Genetic risk factors for folate responsive neural tube defects. Annu Rev Nutr 37:269–91.
Molto J, Kirckpatrick CL, Keron J. 2019. The paleoepidemiology of sacral spina bifida occulta in population samples from the Dakhleh Oasis, Egypt. Int J Paleopathol 26:93–103.
Moore CA, Li S, Li Z, Hong SZ, Gu HQ, Berry RJ, Mulinare J, Erickson JD. 1997. Elevated rates of severe neural tube defects in a high prevalence area of northern China. Am J Med Genet 73:113–8.
Moreira A, Carvalho A, Portugal I, Jesus JM. 2017. Complete dorsal pancreatic agenesis, and unilateral renal agenesis. Radiol Case Rep 13(1):68–71.
Morioka T, Murakamu N, Kanata A, Tsukamoto H, Suzuki S. 2019. Retained medullary cord with sacral subcutaneous meningocele and congenital dermal sinus. Childs Nerv Syst 36:423–7.
Morris JK, Wald NJ. 1999. Quantifying the decline in the birth prevalence of neural tube defects in England and Wales. J Med Screen 6:182–5.
Morrison K, Papapetrou C, Hol FA, Mariman ECM, Lynch SA, Burn J, Edwards YH. 1998. Susceptibility to spina bifida: an association study of five candidate genes. Ann Hum Genet 62:379–96.
Morse D. 1978. Ancient diseases in the mid-west. Illinois State Museum Rep 15(2):181.
Mosiello G, Capitanucci ML, Gatti C, Adorisio O, Lucchetti MC, Silveri M, Schingo PSM, De Gennaro M. 2003. How to investigate neurovesical dysfunction in children with anorectal malformations. J Urol 170(4):1610–3.
Multu H, Kizgut B, Sozer CS, Urker K, Acar O, Erol AS. 2020. Sacral spina bifida occulta: rare occurrence in Byzantine Belentepe population in Mugla, Turkey. A possible case for adequate folic acid intake. Homo 71(3):175–88.
Mushrif-Tripathy V, Rajesh SV, Abhayan GS, Sharma BP, Ajithprasad P. 2018. Anthropological analysis of pre-urban Harappan human skeletal remains from Surkotoda in Kachchh district, Gujarat, India. Bull Deccan Coll Res Institute 78:35–44.
Naffaa L, Irami N, Saade C, Sreedher G. 2017. Congenital anomalies of lumbosacral spine. A pictorial review. J Med Imaging Radiat Oncol 61(2):216–24.
Nakamura Y, Takamuki R, Fujisawa Y, Okiyama N, Watanabe R, Ishitsuka Y, Maruyama H, Ishii Y, Fujimoto M. 2018. Congenital peristernal dermal sinus: a case report and published work review. J Dermatol 45(9):242–3.
Nastoulis E, Karakasi MV, Pavlidis P, Thomaidis V, Fishka A. 2019. Anatomy and clinical significance of sacral variations: a systematic review. Folio Morphol 78(4):651–67.
Nayak PK, Mahapatra AK. 2006. Frontal bone agenesis in a patient of spinal dysraphism. Pediatr Neurosurg 42(3):171–3.
Nazar GB, Casale AJ, Roberts JG, Linden RD. 1995. Occult filum terminale syndrome. Pediatr Neurosurg 23(5):228–35.
Neel JV. 1958. A study of major congenital defects in Japanese infants. Am J Hum Genet 10(4):398–445.
Nicola Z, Antonio C, De Tommasi A. 2014. Cervical dermal sinus complicated with intermedullary abscess in a child: case report and review of the literature. Eur Spine J 23(2):193–6.
O’Connor KP, Smitherman AD, Milton CK, Palejwala AH, Lu VM, Johnston SE, Homburg H, Zhao D, Martin MD. 2020. Surgical treatment of tethered cord syndrome in adults: a systematic review and meta-analysis. World Neurosurg 137:221–41.
O’Neill BR, Gallegos D, Herron A, Palmer C, Stence NV, Hankinson TC, Wilkinson C, Handler MH. 2017. Use of magnetic resonance imaging to detect occult spinal dysraphism in infants. J Neurosurg Pediatr 19:217–26.
O’Neill P, Sign J. 1991. Occult spinal dysraphism in children: need for early neurosurgical referral. Chils Nerv Syst 7(6):309–11.
Ohara K, Nakamura K. 1994. Human tail. Brit J Plast Surg 47(9):288–9.
Orman G, Tijssen MPM, Seyfert D, Gassner I, Huisman TAGM. 2019. Ultrasound to evaluate neonatal spinal dysraphism: a first line alternative to computed tomography and magnetic resonance imaging. J Neuroimaging 29(5):553–64.
Pacheco-Jacome E, Ballesteros MC, Jayakar P, Morrison G, Ragheb J, Medina LS. 2003. Occult spinal dysraphism: evidence-based diagnosis and treatment. Neuroimaging Clin 13(2):327–34.
Page MJ, Moher D. 2017. Evaluations of the uptake and impact of the preferred reporting items for systematic reviews and meta-analyses (PRISM) statement and extensions: a scoping review. Systematic rev 6:263–17.
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffman TC, Mukrow CD, Shamseer L, Tetzlaff JM, Aki EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hrobjartsson A, Laiu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuiness LA, Stewart LA, Thomas J, Triclo AC, Welch VA, Whiting P, McKenzie JE. 2021. PRISMA 2020 expalnation and elaboration: updated guidance and exemplars for reporting systematic reviews. Brit Med J 372:1–36.
Pang D, Dias MS. 1993. Cervical myelomeningoceles. Neurosurg 33(3):363–72.
Pang D. 1995. Surgical complications of open spinal dysraphism. Neurosurg Clin N Am 6(2):243–47.
Papenfuss T, Trautner H, Schwemmer U. 2007. Pediatric anaesthesia for neurological procedures. Anasthesiol Intensive Med Notfallmed Schmerzther 42(6):452–61.
Papp T, Porter RW. 1994. Changes of the lumbar spinal canal proximal to spina bifida occulta. An archaeologic study of clinical significance. Spine 19:1508–11.
Paraskeuas G, Tzika M, Kitsoulis P. 2013. Lumbosacral transition vertebrae associated with sacral spina bifida occulta: a case report. Acta Medica 56(3):126–9.
Patel N, Viguera AC, Baldessarini RJ. 2018. Mood-stabilising anticonvulsants, spina bifida, and folate supllementation: commentary. J Clin Psychopharmacol 38(1):7–10.
Patel ZK, Thummar B, Rathod SP, Singel TC, Patel S, Zalawadia A. 2011. Multicentric morphometric study of dry human sacra of Indian population in Gujarat Region. NJIRM 2(2):37–5.
Peralta CFA, Botelho RD, Romano ER, Imada V, Lamis F, Junior RR, Nani F, Stoeber GH, de Salles AAF. 2020. Fetal open spinal dysraphism repair through mini-hysterotomy: influence of gestational age at surgery on the perinatal outcomes and postnatal shunt rates. Prenat Diag 40(6):689–97.
Petrova JG, Vaktskjold A. 2009. The incidence of neural tube defects in Norway and the Arkhangelskaja Oblast in Russia and the association with maternal age. Acta Obstet Gynecol 88:667–72.
Pierre Kahn A, Zerah M, Renier D, Cinalli G, Sainte-Rose C, Lellouch-Tubiana A, Brunelle F, Le Merrer M, Giusicelli Y, Pichon J, Kleinknecht B, Nataf F. 1997. Congenital lumbosacral limpomas. Childs Nerv Syst 13(6):298–334.
Pietrusewsky M, Douglas MT, Ikehara-Quebral. 1997. An assessment of health and disease in the prehistoric inhabitants of the Mariana Islands. Am J Phys Anthrop 104:315–42.
Piontek J. 1971. Variation in the closure of the sacral canal of man. Folia Microbiol 4:459–464.
Pollard AM, Ditchfield P, Piva E, Wallis S, Falys C, Ford S. 2012. ‘Sprouting like cockle amongst the wheat’: the St Brice’s Day massacre and the isotopic analysis of human bones from St John’s College Oxford. Oxf J Archaeol 31(1):83–102.
Ponti G, Pellacani G, Tomasi A, Summaia G, Manfredini M. 2016. Skeletal stigma as keys to access the composite and ancient Gorlin-Goltz syndrome history; the Egypt, Pompeii and Herculaneum lessons. Gene 589:104–11.
Porter RW, Wicks M, Ottewell D. 1978. Measurement of the spinal canal by diagnostic ultrasound. J Bone Joint Surg 60(4):481–5.
Post RH. 1966. Pilot study: populations differences in the frequency of spina bifida occulta. Eugen Q 13:341–52.
Postoev VA, Nieboer E, Grjibouski AM, Odland JO. 2015. Prevalence of birth defects in an Artic Russian setting from 1973 to 2011: a register-based study. Reprod. Health 12(3):1–8.
Povo A, Arantes M, Matzel KE, Barbosa J, Ferreira MA. 2016. Sacral malformations: use of imaging to optimise sacral nerve stimulation. Int J Colorectal Dis 31(2):351–7.
Prasad GL, Hegde A, Divya D. 2019. Spinal intramedullary abscess secondary to dermal sinus in children. Eur J Pediatr Surg 29(3):229–38.
Proctor MR, Bauer SB, Scott RM. 2000. The effect of surgery for split spinal cord malformation on neurologic and urologic function. Pediatr Neurosurg 32(1):13–19.
QI BQ, Beasley SW, Aesic D. 2004. Abnormalities of the vertebral column and ribs associated with anorectal malformations. Pediatr Surg Int 20(7):529–33.
Rajpal S, Salamat MS, Tubbs RS, Kelly DR, Oakes WJ, Iskander BJ. 2007. Tethering tracts in spina bifida occulta: resisting an established nomenclature. J Neurosurg Spine 7(3):315–22.
Rankin J, Glinianaia S, Brown R, Renwick M. 2000. The changing prevalence of neural tube defects: a population-based study in the north of England, 1984–1996. Pediatr Perinat Epidemiol 14:104–10.
Rankin J, Pattenden S, Abramsky L, Boyd P, Jordan H, Stone D, Vrijheid M, Wellesley D, Dolk H. 2005. Prevalence of congenital anomalies in 5 Bristish regions, 1991–1999. Arch Dis Child Fetal Neonatal Ed 90:374–79.
Rebordosa C, Kogevinas M, Horvath-Puho E, Norgard B, Morales M, Czeizel AE, Vilstrup H, Sorensen HT, Olsen J. 2008. Acetaminophen use during pregnancy: effects on risk for congenital abnormalities. Am J Obstet Gynecol 198(2):178–84.
Relton CL, Wilding CS, Jonas PA, Lynch SA, Tawn EJ, Burn J. 2003. Genetic susceptibility to neural tube defect pregnancy varies with offspring prototype. Clin Genet 64:424–28.
Rentea RM, Halleran DR, Wood RJ, Levitt MA. 2020. The role of laparoscopy in anorectal malformations. Eur J Pediatr Surg 30(2):156–63.
Riley MM, Halliday JL, Lumley JM. 1998. Congenital malformations in Victoria, Australia, 1983–1995: an overview of infant characteristics. J Pediatr Child Health 34:233–40.
Rios L, Kirell TL, Lalueza-Fox C, Estalrrich A, Garcia-Tabernero A, Huguet R, Quintino Y, de la Rasilla M, Rosas A. 2019. Skeletal anomalies in the Neanderthal family of Al Sidron (Spain), support a role in inbreeding in Neanderthal extinction. Sci Rep 9(1):1697–708.
Robinson AJ, Russell S, Rimmer S. 2005. The value of ultrasonic examination of the lumbar spine in infants with specific reference to cutaneous markers of occult spinal dysraphism. Clin Radiol 60:72–77.
Robinson RO, Lippold T, Land R. 1986. Body schema: does it depend on bodily-derived sensations? Dev Med Child Neurol 28(1):49–52.
Roche MB, Rowe GG. 1952. The incidence of separate neural arch and coincident bone variations. J Bone Joint Surg 34(2):491–493.
Rochtus A, Winand R, Laenen G, Vangeel E, Izzi B, Wittevrongel C, Moreau Y, Verpoorten C, Jansen K, Van Greet C, Freson K. 2016. Methylome analysis for spina bifida shows SOX18 hypomethylation as a risk factor with evidence for complex (epi)genetic interplay to affect neural tube development. Clin Epigenet 8:108–20.
Rodriguez JI, Garcia M, Morales C, Morillo A, Delicado A. 1990. Trisomy 13 syndrome and neural tube defects. Am J Med Genet 36(4):513–6.
Rosano A, Smithells D, Cacciani L, Botting B, Castilla E, Cornel M. 1979. Time trends in neural tube defect prevalence in relation to predictive strategies: an international study 53(10):630–5.
Ruggieri M, Polizzi A, Catanzaro S, Bianco ML, Pratico AD, Di Rocco C. 2020. Neurocutaneous melanocytosios (melanosis). Childs Nerv Syst 36(10):2571–96.
Ruhli FJ, Galassi FM, Haeusler M. 2016. Palaeopathology: current challenges and medical impact. Clin Anat 29:816–22.
Ruhli FJ, Henneberg M. 2013. New perspectives on evolutionary medicine: the relevance of microevolution for human health and disease. BMC Med 11:115–22.
Ruhli FJ, Solomon LB, Henneberg M. 2003. High prevalence of tarsal coalitions and tarsal joint variants in a recent cadaver sample and its possible significance. Clin Anat 16:411–5.
Ruiz-Osuna C, Avila-Zamorano ML, Suraez-Ahedo C, Trueba-Davalillo C. 2009. Association of intercalary cervical bone and occult lumbar and sacral spina bifida. Case report. Acta orthop Mex 23(1):31–4.
Sade B, Beni-Adani L, Ben-Sira L, Constantini S. 2003. Progression of terminal syrinx in occult spina bifida after untethering. Childs Nerv Syst 19:106–8.
Sairyo K, Goel VK, Vadapalli S, Vishnubhotla SL, Biyani A, Ebraheim N, Terai T, Sakai T. 2006. Biomechanical comparison of lumbar spines with or without spina bifida occulta. A finite element analysis. Spinal Cord 44:440–44.
Sakakibara R, Hattori T, Uchiyama T, Kamura K, Yamanishi T. 2003. Uroneurological assessment of spina bifida cystica and occulta. Neurourol Urodyn 22(4):328–34.
Saluja PG. 1988. The incidence of spina bifida occulta in a historic and a modern London population. J Anat 158:91–93.
Sanabria Sanchinel AA, Lin Y, Rodriguez Rubio D. 2020. Pseudomeningocele: headache, apnoea and syncope. Neurologica (Engl Ea) 36(8):654–56.
Saniotis A, Henneberg M. 2011. Medicine could be constructing human bodies in the future. Med Hypoth 77:560–64.
Sardana K, Gupta R, Garg VK, Mishra D, Mishra P, Grover C, Mendiratta V. 2009. A prospective study of cutaneous manifestations of spinal dysraphism from India. Pediatr Dermatol 26(6):688–95.
Sarin Y. 2013. Cutaneous stigmata of occult stigmata dysraphism. J Neuronatal Surg 2(1):15.
Sarwan S, Rampersad B. 2012. Pyourachus in spina bifida: a case report and review. Urol 80(2):427–9.
Sato N, Sato H. 2000. Diastematomyelia. Ryoikibetsu Shokogun Shirizu 28(3):387–390.
Sattar MT, Bannister CM, Turnbull IW. 1996. Occult spinal dysraphism – the common combination of lesions and the clinical manifestations in 50 paitents. Eur J Pediatr Surg 6(1):10–4.
Savona-Ventura C. 2007. Congenital malformation: a historical perspective in a Mediterranean community. Malta Med J 19(1):52–55.
Sawin KJ, Brei TJ, Houtrow AJ. 2020. Quality of life: guidelines for the care of people with spina bifida. J Pediatr Rehabil Med 13(4):565–82.
Sayed AR, Bourne D, Pattinson R, Nixon J, Henderson B. 2008. Decline in the prevalence of neural tube defects following folic acid fortification and its cost benefit in South Africa. Birth Defects Res 82:211–6.
Scatliff JH, Kendall BE, Kingsley DPE, Britton J, Grant DN, Hayward RD. 1988. Closed spinal dysraphism: analysis of clinical, radiological, and surgical findings in 104 consecutive patients. Am J Rad 152:1049–57.
Schindelmann KH, Paschereit F, Steege A, Stoltenburg-Didinger, Kaindle AM. 2021. Systematic classification of spina bifida. J Neuropathol Exp Neurol 80(4):294–305.
Schmidt C, Bryant E, Iwanaga J, Oskouian RJ, Oakes WJ, Tubbs RS. 2017. Meningocele manque: a comprehensive review of this enigmatic finding in occult spinal dysraphism. Childs Nerv Syst 33:1065–71.
Schmidt SM, Robinson B, Jones DA. 1990. The tethered spinal cord. Etiology and clinical manifestations. Orthop Rev 19(10):870–6.
Schropp C, Sorensen N, Collmann H, Kraub J. 2006. Cutaneous lesions in occult spinal dysraphism – correlation with intraspinal findings. Childs Nerv Syst 22:125–31.
Schwartz ES, Rossi A. 2015. Congenital spine anomalies: the closed spinal dysraphism. Pediatr Radiol 45(3):413–9.
Schweitzer ME, Balsam D, Weiss R. 1992. Spina bifida occulta: incidence in parents of offspring with spina bifida cystica. Spine 18:785–6.
Sebold CD, Melvin EC, Siegel D, Mehltretter L, Enterline DS, Nye JS, Kessler J, Bassuk A, Speer MC, George TM. 2005. Recurrence risks for neural tube defects in siblings of patients with lipomyelomeningocele. Genet Med 7(1):64–7.
Sepulveda W, Wang AE, Sepulveda F, Alcalde JL, Pevoto JL, Otayza F. 2017. Prenatal diagnosis of spina bifida: from intracranial translucency to intrauterine surgery. Childs Nerv Syst 33(7):1083–99.
Serna MJ, Vazquez-Doual J, Vanaclocha V, Zubieta JL, Quintanilla E. 1993. Occult spinal dysraphism: a neurosurgical problem with a dermatologic hallmark. Pediatr Dermatol 10(2):149–52.
Sewell MJ, Chiu YE, Drolet BA. 2015. Neural tube defects: review of cutaneous markers and imaging. Pediatr Dermatol 32(2):161–70.
Shin M, Besser LM, Siffel C, Kucik JE, Shaw GM, Lu C, Correa A. 2010. Prevalence of spina bifida among children and adolescents in 10 regions in the United States. Pediatr (Evanston) 126(2):274–79.
Shin SH, Im YJ, Lee M, Lee YS, Choi EK, Han SW. 2013. Spina bifida occulta: not to be overlooked in children with nocturnal enuresis. Int J Urol 20(8):831–5.
Shore LR. 1930. Abnormalities of the vertebral column in a series of skeletons of Bantu natives of South Africa. J Anat 64:206–38.
Shore LR. 1936. Some examples of disease of the vertebral column found in skeletons of ancient Egypt. A contribution and paleopathology. Brit J Surg 24(94):256–71.
Shurtleff DB, Duguay S, Duguay G, Moskowitz D, Weinberger E, Roberts T, Loeser J. 1997. Epidemiology of tethered cord with meningomyelocele. Eur J Pediatr Surg 7(1):7–11.
Silva-Pinto V, Arriaza B, Standen V. 2010. Spina bifida occulta associated with environmental arsenic exposure in a prehispanic sample from northern Chile. Rev Med Chil 138(4):461–169.
Simalcsik A, Miu G, Groza VM, Simalcsik RD. 2011. Regarding occult spinal dysraphism (spina bifida occulta), focussing especially on a medieval population from Isai. Biol Anim 62:131–41.
Simpson D, Baral R, Lee D, Sutherland M, Malik R. 2011. Dermoid sinus in Burmese cats. J Small Anim Pract 52(11):616.
Simriti, Singh N, Dev B, Raina S. 2017. Variation in morphometry of dry human sacral hiatus. JK Sci 19:161–164.
Sims-Williams HJ, Sims-Williams HP, Kabachelor EM, Warf BC. 2017. Quality of life among children with spina bifida in Uganda. Arch Dis Child 102:1057–61.
Singh DK, Kumar B, Sinha VD, Bagaria HR. 2008. The human tail: rare lesion with occult spinal dysraphism – a case report. J Pediatr Surg 43:41–3.
Smithells RW, Chinn ER. 1965. Spina bifida in Liverpool. Dev Med Child Neurol 7:258–68.
Smithells RW, Sheppard S, Wild J. 1989. Prevalence of neural tube defects in the Yorkshire region. Community Med 11(2):163–7.
Sneineh A, Kareem A, Gabos P, Keller MS, Bowen JR. 2002. Ultrasonography of the spine in neonates and young infants with a sacral skin dimple. J Pediatr Orthop 27(6):761–2.
Solomon LB, Ruhli FJ, Lee YC, Henneberg M. 2009. Secular trend in the opening of the sacral canal. Spine 34:244–8.
Soonwala N, Overweg-Plandsoen WCG, Brouwer OF. 1999. Early clinical signs and symptoms of occult spinal dysraphism: a retrospective study of 47 paitents. Clin Neurol Neurosurg 101:11–4.
St Louis AM, Kim K, Browne ML, Liu G, Liberman RF, Nembhard WN. 2017. Prevalence trends of selected major birth defects: a multi-state population-based study, United States, 1999–2007. Birth Defects Res 109:1442–50.
Steinbok P. 1995. Dysraphic lesions of the cervical spinal cord. Neurosurg Clin North Am 6(2):367–76.
Stewart TD. 1932. The vertebral column of the Eskimo. Am J of Anthropol 17:123–136.
Steyn M, Iscan MY. 2008. Matruc sex determination from the pelvis in modern Greeks. Forens Sci Intl 179:86–92.
Strubbe EH, Lemmens JAM, Thijn CJP, Willemsen WNP, Van-Toor BSJ. 1992. Spinal anomalies and the atypical form of the Mayer-Rokitansky-Kuster-Hauser Syndrome. Skel Radiol 21:459–62.
Sunderland R, Emry JL. 1979. The mortality and birth rates of spina bifida during a period of treatment, selection, and antenatal screening in Sheffield, 1963–1978. Z Kinerchir Grenzgeb 28(4):294–301.
Sung HJ, Lee HS. 2019. Dorsal midline cutaneous stigmata associated with occult spinal dysraphism in pediatric patients. Korean J Pediatr 62(2):68–74.
Sutow WW, Pryde AW. 1955. Incidence of spina bifida occulta in relation to age. Am J Dis Child 90: 211–7.
Swallow DM. 2003. Genetics of lactase persistence and lactose intolerance. Ann Rev Genet 37:197–219.
Szabo N, Gerger G, Valek A, Eller J, Kaiser L, Sztriha L. 2013. Birth prevalence of neural tube defects: a population-based study in south-eastern Hungary. Childs Nerv Syst 29:621–7.
Tan KB, Tan SH, Tan KH, Yeo GS. 2007. Anencephaly in Singapore: a ten-year series, 1993–2003. Singapore Med J 48(1):12–5.
Tardieu GG, Loukas M, Fisahn C, Shoja MM, Oskouian RJ, Tubbs RS. 2017. The Italian Giuseppe Muscatello (1866–1951) and his contributions to our understanding of childhood spina bifida aperta and occulta. Childs Nerv Syst 33:389–91.
Tawfik S, Phan K, Mobbs RJ, Rao PJ. 2020. The incidence of pars interarticularis defect in athletes. Global Spine J 10(1):89–101.
Thompson DNP. 2014. Spinal dysraphic anomalies; classification, presentation, and management. Pediatr Child Health 24(10):431–8.
Thuy M, Chaseling R, Fowler A. 2015. Spinal cord tethering procedures in children: a 5-year retrospective cohort study of the early postoperative course. J Clin Neurosci 22(5):838–42.
Timbolschi D, Schaefer E, Monga B, Fattori D, Dott B, Favre R, Kohler M, Nisand I, Viville B, Astruc D, Kehril P, Gasser B, Linder V, Marcellin L, Flori E, Girrard-Lemaire F, Dollfus H, Doray B. 2015. Neural tube defects: the experience of the registry of congenital malformations of Alsace, France, 1995–2009. Fetal Diagn Ther 37(1):6–17.
Tomaszewska A, Kwiatkowska B. 2019. Non-metric traits, physiological stress indicators and paleopathological lesion in human skeletal remains from an early modern cemetery in Wyszynski Street, Wroclaw, Poland. (15th–18th centuries AD). Anthropol Rev 82(2):191–202.
Tortori-Donati P, Cama A, Rosa ML, Andreussi L, Taccone A. 1990. Occult spinal dysraphism: neuroradiological study. Neuroradiol 31:512–22.
Trapp B, De Andrade Lourencao Freddi T, Oliveira Morais Hans M, Fonseca Teixeira Lemos Calixto I, Fujino E, Alves Rojas LC, Burlin S, Cerqueira Costa DM, Carete H Jr, Abdala N, Tobaru Tibana LA, Takashi Takenhara E, Gomez GD. 2021. A practical approach to diagnosis of spinal dysraphism. Radiographics 41(2):559–75.
Tu A, Steinbok P. 2013. Occult tethered cord syndrome: a review. Childs Nerv Syst 29(9):1635–40.
Tubbs RS, Oakes WJ. 2006. A simple method to deter retethering patients with spinal dysraphism. Childs Nerv Syst 22(7):715–6.
Tubbs RS, Wellons JC 3rd, Iskander BJ, Oakes WJ. 2004. Isolated flat capillary midline lumbosacral hemangiomas as indicators of occult spinal dysraphism. J Neurosurg 100(2):86–9.
Tuite GF, Thomson DNP, Austin PF, Baver SB. 2018. Evaluation and management of tethered cord syndrome in occult spinal dysraphism: recommendations from the international childrences continence society. Neurourol Urodyn 37(3):890–903.
Ucar DH, Omeroglu H, Eren A, Inan M, Baktir A, Aksoy MC, Omeroglu S. 2003. Occult spinal dysraphism and its association with hip dysplasia in females. Int Orthop 27(2):70–2.
Uff C, Bradford R. 2005. Retrograde intraventricular haemorrhage caused by a traumatic sacral pseudomeningocele in the presence of spina bifida occulta. Case report. J Neurosurg Spine 3(5):390–2.
Ulizzi L, Astolfi P, Zonta LA. 1998. Natural selection in industrialised countries: a study of 3 generations of Italian newborns. Ann Hum Gen 62:47–53.
Urrutia J, Cuellar J, Zamora T. 2014. Spondylolysis and spina bifida occulta in pediatric patients: prevalence study using computed tomography as a screening method. Eur Spine J 25:590–5.
Valentini LG, Selvaggio G, Erbetta A, Cordella R, Pecoraro MG, Bova S. 2013. Occult spinal dysraphism: lesson learned by retrospective analysis of 149 surgical cases about natural history, surgical indications, urodynamic testing and intraoperative neurophysiological monitoring. Childs Nerv Syst 29:1657–69.
Van de Vijner K. 2018. Past life and death in a Felmish town. An archaeo-anthropological study of burials from the medieval and post-medieval St Rombout’s Cemetery in Mechelen, Belgium (10th-18th century). J Arch Sci Rep 20:524–55.
Vantankhah S, Jalilvand M, Sarkhosh S, Azarmi M, Mohseni M. 2017. Prevalence of congenital anomalies in Iran: a review article. Iran J Public Health 46(6):733–43.
Varshney G, Gupta DK. 2020. Giant terminal myelocystocele: a case report. J Pediatr Neurosci 15(3):286–9.
Veena K, Palaksha HK. 1999. Incidental Spina bifida occulta and functional enuresis observed during reflex therapy. J Child Neurol 14(8):541–3.
Veenboer PW, De Kort LM, Chrzan RJ, De Jong TP. 2015. Urinary considerations for adult patients with spinal dysraphism. Nat Rev Urol 12(6):331–9.
Venkataramana NK. 2011. Spinal Dysraphism. J Pediatr Neurosci 6(1):31–40.
Vintzileos AM, Campbell WA, Weinbaum PJ, Nochimson DJ. 1987. Perinatal management and outcome of fetal ventriculomegaly. Obstet Gynecol 69(1):5–11.
Vishal K, Vinay KV, Remya K, Arunachalam K, Shishir K. 2012. High sacral hiatus with non-fusion of lamina of first sacral vertebrae: a case report. Nitte Uni J Health Sci 2(4):60–2.
Wals PD, Trochet C, Pinsonneault L. 1999. Prevalence of neural tube defects in the Province of Quebec, 1992. Canad J Public Health 90(4):237–9.
Wang T, Fielding LC, Parikh A, Kothari M, Alamin T. 2015. Sacral spinous processes: a morphologic classification and biomechanical characterisation of strength. Spine J 15:2544–51.
World Health Organisation. 2012. Life health status statistics: mortality.
World Health Organisation. 2020. Life expectancy and healthy life by country.
Williams LJ, Mai CT, Edmonds LD, Shaw GM, Kirby RS, Hobbs CA, Sever LF, Miller LA, Meaney FJ, Levitt M. 2002. Prevalence of spina bifida and anencephaly during the transition to folic acid fortification in the United States. Teratol 66:33–9.
Williams LJ, Rasmussen SA, Flores A, Kirby RS, Edmonds LD. 2005. Decline in the prevalence of spina bifida and anencephaly by race/ethnicity: 1995–2002. Pediatr 116(3):580–6.
Willis TA. 1923. The lumbo-sacral vertebral column in man, its stability, form, and function. Am J Anat 32:95–123.
Wilson SN, Kongnyuy M, Joseph DB, Wilson TS. 2021. Urodynamic utilisation in the adult spina bifida patient. An institutional review. J Pediatr Rehabil Med 14(4):655–9.
Wolpowitz A. 1978. Spina bifida occulta. S Afr Med J 53(16):614.
Wu JW, Xing YR, Wen YB, Li TF, Xie JF, Feng QD, Shang XP, Li YL, Feng JJ, Wang XX, Zhai RQ, He XF, Chen T, Liu XJ, Wen JG. 2016. Prevalence of spina bifida occulta and its relationship with overactive bladder in middle-aged and elderly Chinese people. Int Neurouro J 20:151–8.
Wu LP, Li YK, Li YM, Zhang YQ, Zhong SZ. 2009. Variable morphology of the sacrum in a Chinese population. Clin Anat 22(5):619–26.
Xiao CG. 2012. Xiao procedure for neurogenic bladder in spinal cord injury and spina bifida. Neurogenic Bladder 7:83–7.
Xiong Y, Yang L, Zhen W, Fangyong D, Feng W, Ting L. 2018. Conservative, and surgical treatment of pediatric asymptomatic lumbosacral lipoma: a meta-analysis. Neurosurg Rev 41(3):737–43.
Yamanda S, Won DJ, Pezeshkpour G, Yamanda BS, Yamanda SM, Siddiqi J, Zouros A, Colohan ART. 2007. Pathophysiology of tethered cord syndrome and similar complex disorders. Neurosurg Focus 23(2):6–16.
Yameogo SP, Ghedira K. 2019. Occipital dermal sinus: the tip of the iceberg. J Pediatr 204:314.
Yuan X, Chang W, Hou A, Wang W, Zhang S, Liu D, Gao F, Li H, Wang W. 2008. Constipation associated with spina bifida occulta in children. Clin Gastroenterol Hepatol 6(12):1348–53.
Yue WM, Bridner W, Gaines RW. 2005. Abnormal spinal anatomy in 27 cases of surgically corrected spondyloptosis: proximal sacral endplate damage as a possible cause of spondyloptosis. Spine (Phila Pa 1976) 30(6):22–6.
Yuskiv N, Andelin CO, Polischuk S, Shevchuk U, Sosynyuk Z, Vihovska T. 2004. High rates of neural tube defects in Ukraine. Birth Defects Res 70:400–2.
Zaganjor I, Sekkarie A, Tsang SL, Williams J, Razzaghi H, Mulinare J, Sniezek JE, Cannon MJ, Rosenthal J. 2016. Describing the prevalence of neural tube defects worldwide: a systematic literature review. Plos One 11(4):1–31.
Zemirline A, Vincent JP, Sid-Ahmed S, Nen DL, Dubrana F. 2013. Lumbo-sacral malformations and spina bifida occulta in medieval skeletons from Brittany. Eur J Orthop Surg Traumatol 23:149–53.
Zhang TN, Gong TT, Chan YL, Wu QJ, Zhang Y, Jiang CZ, Li J, Li LL, Zhou C, Huang YH. 2017. Time trends in the prevalence and epidemiological characteristics of neural tube defects in Liaoning Province, China, 2006–2015: a population-based study. Oncotarget 8(10):17092–104.
Zimmerman MR. 1977. The mummies of the tomb of Nebwenenef: paleopathology and archaeology. J Am Res. Centre Egypt 14:33–6.
Supplementary Material
Table 6. Results of the initial screening process. Inclusion/exclusion criteria and number of publications included/excluded by database outlined
| Exclusion reason | Number Excluded: Embase (n=) |
Number Excluded: PubMed (n=) | Number Excluded: Adelaide Library (n=) |
Number Excluded: Google Scholar (n=) |
Total (n=) /409 |
| Duplicates | 9 | 2 | 1 | 1 | 13 |
| Case Studies | 11 | 5 | 1 | 1 | 17 |
| Surgical Texts | 12 | 13 | 2 | 27 | |
| Responses/Abstracts/Reviews | 1 | 1 | |||
| Radiographical Methods | 6 | 3 | 2 | 11 | |
| Ethics | 1 | 1 | |||
| Non-Human Studies | 1 | 3 | 1 | 5 | |
| General no % data | 6 | 16 | 1 | 3 | 26 |
| SBC not SSBO | 10 | 13 | 10 | 5 | 38 |
| Unrelated Clinical Conditions | 26 | 25 | 16 | 2 | 68 |
| Nonrandomised | 11 | 7 | 4 | 2 | 24 |
| Total Included | 08/100 | 12/100 | 103/141 | 53/68 | 178 |
| Total Excluded | 92/100 | 88/100 | 38/141 | 15/68 | 231 |
Table 7. Results of the internal validity screening process. Inclusion/exclusion criteria and number of publications included/excluded by database outlined
| Exclusion Criterion | Publications Excluded (n=) |
| Duplicate data | 3 |
| Unreliable data | 2 |
| Case Studies | 5 |
| Surgical Texts | 3 |
| SBC not SSBO | 55 |
| No segment data | 5 |
| No S1 data | 6 |
| Nonrandomised | 15 |
| Non-Human | 2 |
| Radiograph method | 7 |
| General no % data | 10 |
| Unrelated condition | 26 |
| Total Included | 39/178 |
| Total Excluded | 139/178 |
Table 8. Included studies with sample size, date of samples as well as characteristics and any necessary assessments for bias in each publication
| (n=) | Publication | Sample Size + Date |
Characteristics | Risk of Bias Assessment* |
| 1 | Zemirline A et al. Lumbo-sacral malformations and spina bifida occulta in medieval skeletons from Brittany. Eur J Orthop Surg Traumatol. 2013;23: 149–153. | 30 768 CE |
Archaeological study of recovered skeletal human remains. |
Anecdotal data Clear, accurate and included segment data for SSBO, S1 |
| 2 | Molto JE, et al. The paleoepidemiology of sacral spina bifida occulta in population samples from the Dakhleh Oasis, Egypt. Int J Palaeopathol. 2019;26: 93–103. | 116 116 BCE |
Archaeological study of recovered human remains. (Dry human sacra) |
Well-structured numerical results Clear, accurate and included segment data for SSBO, S1 |
| 3 | Urrutia J, et al. Spondylolysis and spina bifida occulta in paediatric patients. Prevalence study using computed tomography as a screening method. Eur Spine J. 2016;25: 590–595. | 228 2005 CE |
Radiographic study of live patients with associated pathology. | CT and well-structured numerical results. SSBO and Spondylolysis data separate Clear, accurate and included segment data for SSBO, S1 |
| 4 | Saluja PG. The incidence of spina bifida occulta in a historic and a modern London population. J Anat. 1988;158: 91–93. | 112 1816 CE |
Archaeological study of recovered human remains. (Dry human sacra) |
Well-structured numerical results Clear, accurate and included segment data for SSBO, S1 |
| 5 | Lee YC et al. Confirmation of microevolutionary increase of spina bifida occulta among Swiss birth cohorts. Eur Spine J. 2011;20: 776–780. | 384 1965 CE |
Radiographic study of birth cohorts. Anonymised CT data. | Well-structured numerical results Clear, accurate and included segment data for SSBO, S1 |
| 6 | Ali S, et al. The prevalence of spina bifida occulta in a Pakistani population: a study of dry human sacra. Anaesth, Pain Intensive Care. 2014;18: 157–161. | 200 1954 CE |
Modern anatomical study of archived dry human sacra. | Well-structured numerical results Clear, accurate and included segment data for SSBO, S1 |
| 7 | Shin SH et al. Spina bifida occulta: not to be overlooked in children with nocturnal enuresis. Int J Urol. 2013;20: 831–835. | 160 1999 CE |
Radiographic study in live patients with associated pathology. | Well-structured numerical results. SSBO and enuresis data separated Clear, accurate and included segment data for SSBO, S1 |
| 8 | Wu L et al. Variable morphology of the sacrum in a Chinese population. Clin Anat. 2009;22: 619–626. | 203 1961 CE |
Modern anatomical study of archived dry human sacra. | Well-structured numerical results Clear, accurate and included segment data for SSBO, S1 |
| 9 | Cakiroglu B et al. The adverse influence of spina bifida occulta on the medical treatment outcome of primary monosymptomatic nocturnal enuresis. Archive Italian Urol. 2014;86: 270–273. | 233 1999 CE |
Radiographic study in live patients with associated pathology. | Well-structured numerical results Clear, accurate and included segment data for SSBO, S1 |
| 10 | Solomon LB et al. Secular trend in the opening of the sacral canal: An Australian study. Spine. 2009;34: 244–248. | 200 1945 CE |
Radiographic study of birth cohorts. Anonymised CT data. | Well-structured numerical results Clear, accurate and included segment data for SSBO, S1 |
| 11 | El-Din A et al. Congenital anomalies of the vertebral column: a case study on ancient and modern Egypt. Int J Osteoarchaeol. 2006;16: 200–207. | 270 2424 BCE |
Archaeological study of recovered human remains. |
Well-structured numerical results Clear, accurate and included segment data for SSBO, S1 |
| 12 | Maat GJ et al. Analysis of human skeletons from a Hellenistic period, buried at a ruined Bronze Age building on Failaka, Kuwait. Maison de l’Oreint. 1990;18: 85–102. | 12 1770 BCE |
Archaeological study of recovered human remains. (Dry human sacra) |
Well-structured numerical results Clear, accurate and included segment data for SSBO, S1 |
| 13 | Kim DW et al. Morphological diversities of sacral canal in children: three-dimensional computed tomography study. Korean J Pain. 2014;27: 253–259. |
143 1996 CE |
Radiographic study in live patients with associated pathology. | Well-structured numerical results. SSBO data and other anomaly data separated >Clear, accurate and included segment data for SSBO, S1 |
| 14 | Wu JW et al. Prevalence of spina bifida occulta and its relationship with overactive bladder in middle-aged and elderly Chinese people. Int Neurouro J. 2016;20: 151–158. | 1061 1954 CE |
Radiographic study in live patients with associated pathology. | Well-structured numerical results. SSBO and bladder dysfunction data separated Clear, accurate and included segment data for SSBO, S1 |
| 15 | Fidas A et al. Prevalence and patterns of spina bifida occulta in 2707 normal adults. Clin Rad. 1987;38: 537–542. | 2707 1911 CE |
Radiographic study in live patients with associated pathology. | Well-structured numerical results Clear, accurate and included segment data for SSBO, S1 |
| 16 | Shore LR. Abnormalities of the vertebral column in a series of skeletons of Bantu natives of South Africa. J Anat. 1930;64: 206–238. | 155 1945 CE |
Archaeological study of recovered human remains. (Dry human sacra) |
Anecdotal data Clear, accurate and included segment data for SSBO, S1 |
| 17 | Masnicova S et al. Developmental anomalies in skeletal remains from the great Moravia and Middle Ages cemeteries at Devin, (Slovakia). Intl J Osteoarchaeol. 2003;13: 266–274. | 150 1115 BCE |
Archaeological study of recovered human remains. (Dry human sacra) |
Well-structured numerical results Clear, accurate and included segment data for SSBO, S1 |
| 18 | Hussien FH et al. Spinal pathological findings in ancient Egyptians of the Greco-Roman period living in Bahriyah Oasis. Int J Osteoarchaeol. 2009;19: 613–627. | 77 289 BCE |
Archaeological study of recovered human remains. (Dry human sacra) |
Anecdotal data Clear, accurate and included segment data for SSBO, S1 |
| 19 | Mays S. Spondylolysis, spondylolisthesis, and lumbo-sacral morphology in a medieval English skeletal population. Am J Phys Anthropol. 2006;131: 352–362. | 422 1465 CE |
Archaeological study of recovered human remains. (Dry human sacra) |
Well-structured numerical results. SSBO and Spondylolysis data separate Clear, accurate and included segment data for SSBO, S1 |
| 20 | Kim Y et al. Lumbosacral defects in a 16th – 18th century Joseon Dynasty skeletal series from Korea. Biomed Res Int. 2018;28: 1–8. | 198 1666 CE |
Archaeological study of recovered human remains. (Dry human sacra) |
Well-structured numerical results Clear, accurate and included segment data for SSBO, S1 |
| 21 | Schweitzer ME et al. Spina bifida occulta: incidence in parents of offspring with spina bifida cystica. Spine. 1992;18: 785–786. | 177 1932 CE |
Radiographic study in live patients with associated pathology. | Anecdotal data Clear, accurate and included segment data for SSBO, S1 |
| 22 | McGrath M et al. Anatomical observations related to radiological findings in spina bifida -occulta of the lumbo-sacral spine. J Osteopath Med. 2004;7: 70–78. | 40 1994 CE |
Radiographic study specifically designed for SSBO. | Anecdotal data Clear, accurate and included segment data for SSBO, S1 |
| 23 | Papp T et al. Changes of the lumbar spinal canal proximal to spina bifida occulta. An archaeologic study of clinical significance. Spine. 1994;19: 1508–1511. | 104 367 CE |
Archaeological study of recovered human remains. (Dry human sacra) |
Well-structured numerical results Clear, accurate and included segment data for SSBO, S1 |
| 24 | Jankauskas R. Variations and anomalies of the vertebra column in Lithuanian palaeoosteological samples. Anthropol. 2001;39: 33–38. | 633 1467 CE |
Archaeological study of recovered human remains. (Dry human sacra) |
Well-structured numerical results Clear, accurate and included segment data for SSBO, S1 |
| 25 | Merbs CF. Sagittal clefting of the body and other vertebral development errors in Canadian Inuit skeletons. Am J Phys Anthropol. 2004;123: 236–249. | 218 1867 CE |
Archaeological study of recovered human remains. (Dry human sacra) |
Well-structured numerical results. SSBO and other anomaly data separated Clear, accurate and included segment data for SSBO, S1 |
| 26 | Stewart TD. The vertebral column of the Eskimo. Am J of Anthropol. 1932;17: 123–136. | 217 1990 CE |
Archaeological study of recovered human remains. (Dry human sacra) |
Anecdotal data Clear, accurate and included segment data for SSBO, S1 |
| 27 | Eubanks J et al. Prevalence of sacral spina bifida occulta and its relationship to age, sex, race, and the sacral table angle. Spine. 2009;34: 1539–1543. | 2866 1885 CE |
Radiographic study specifically designed for SSBO. | Well-structured numerical results Clear, accurate and included segment data for SSBO, S1 |
| 28 | Sutow WW et al. Incidence of spina bifida occulta in relation to age. Am J Dis Child. 1955;90: 211–217. | 540 1921 CE |
Radiographic study specifically designed for SSBO. | Well-structured numerical results Clear, accurate and included segment data for SSBO, S1 |
| 29 | Albrecht TL et al. Radiographical method to access the prevalence of sacral spina bifida occulta. Clin Anat. 2007;20: 170–174. | 53 1937 CE |
Radiographic study specifically designed for SSBO. | Well-structured numerical results Clear, accurate and included segment data for SSBO, S1 |
| 30 | Karlin IW. Incidence of spina bifida occulta in children with and without enuresis. Am J Dis Child. 1935;3: 374–393. | 75 1840 CE |
Radiographic study in live patients with associated pathology. | Anecdotal data Clear, accurate and included segment data for SSBO, S1 |
| 31 | Jozsa L et al. The occurrence of spina bifida occulta in medieval and contemporaneous Hungarian populations. Anthropol Hunarica. 1992;22: 51–60. | 233 1328 CE |
Archaeological study of recovered human remains. (Dry human sacra) + Radiographic study specifically for SSBO. |
Anecdotal data Clear, accurate and included segment data for SSBO, S1 |
| 32 | Avrahami E et al. Spina bifida occulta of S1 is not an innocent finding. Spine. 1994;19: 12–15. | 1200 1949 CE |
Radiographic study specifically designed for SSBO. | Well-structured numerical results Clear, accurate and included segment data for SSBO, S1 |
| 33 | Piontek J. Variation in the level of closure in the sacral canal of man. Folia Microbiol. 1971;4: 459–464. | 316 1911 CE |
Modern anatomical study of archived dry human sacra. | Well-structured numerical results Clear, accurate and included segment data for SSBO, S1 |
| 34 | Kubauat DM et al. A study of non-fusion of laminae of the first sacral vertebrae in Western India. Int J Recent Trends Sci Tech. 2013;6: 122–124. | 302 1953 CE |
Archaeological study of recovered human remains. (Dry human sacra) |
Anecdotal data Clear, accurate and included segment data for SSBO, S1 |
| 35 | Groza VM et al. Frequency of spina bifida occulta and other occult spinal dysraphism’s in the medieval population of Isas city: skeleton palaeopathology in the necropolis discovered in the eastern part of the Princely Court, 17th century. Biol Anim. 2012;58: 195–204. | 28 1660 CE |
Archaeological study of recovered human remains. (Dry human sacra) |
Anecdotal data Clear, accurate and included segment data for SSBO, S1 |
| 36 | Henneberg RJ et al. Variation in the closure of the sacral canal in the skeletal sample from Pompeii, Italy, 79AD. Perspect Hum Bio. 1999;4: 177–188. | 124 79 CE |
Archaeological study of recovered human remains. (Dry human sacra) |
Well-structured numerical results Clear, accurate and included segment data for SSBO, S1 |
| 37 | Singh R. Classification causes and clinical implications of sacral spina bifida occulta in Indians. Basic Sci Med. 2013;2: 14–20. | 140 1953 CE |
Archaeological study of recovered human remains. (Dry human sacra) |
Anecdotal data Clear, accurate and included segment data for SSBO, S1 |
| 38 | Al-Dahhan MH et al. Evaluation of spina bifida occulta in young patients presented with lower back pain. Eur J Mol Clin Med. 2020;10: 4416–4422. | 180 2016 CE |
Radiographic study in live patients with associated pathology. | Well-structured numerical results Clear, accurate and included segment data for SSBO, S1 |
| 39 | Kumar P et al. Spina bifida occulta in functional enuresis. Indian J Paediatr. 2005;72: 223–225. | 48 1997 CE |
Radiographic study in live patients with associated pathology. | Well-structured numerical results Clear, accurate and included segment data for SSBO, S1 |
Table 9. Raw data from frequency analysis
| Publication | Date | Total SS | Male SS | Female SS | S1-S5 | S1 | S1-S2 | S1-S3 |
| 1. El-Din and El Banna 2006 | -2424 | 270 | 0.74 | |||||
| 135 | 0.74 | |||||||
| 135 | 0.74 | |||||||
| 2. Maat et al. 1990 | -1770 | 12 | 8.30 | |||||
| 3. Molto et al. 2019 | -874 | 116 | 5.17 | 13.79 | 0.86 | |||
| 64 | 7.80 | 10.93 | 1.56 | |||||
| 52 | 1.92 | 17.30 | ||||||
| -860 | 77 | 2.59 | ||||||
| -868 | 193 | 3.10 | 9.32 | 0.51 | ||||
| 23 | 8.69 | |||||||
| 1766 | 144 | 11.11 | ||||||
| 4. Hussein et al. 2009 | -289 | 77 | 54.54 | |||||
| 41 | 51.21 | |||||||
| 124 | 119 | 35 | 60.00 | |||||
| 124 | 119 | 4.2 | 16.80 | 3.36 | ||||
| 56 | 7.4 | 19.64 | 3.57 | |||||
| 110 | 130 | 63 | 1.58 | 14.28 | 3.17 | |||
| 110 | 130 | 0.76 | 6.15 | 3.84 | 0.76 | |||
| 47 | 2.12 | 6.37 | 8.51 | |||||
| 83 | 6.02 | 1.20 | 1.20 | |||||
| Publication | Date | Total SS | Male SS | Female SS | S1-S5 | S1 | S1-S2andS4-S5 | S1andS3-S5 |
| 5. Mays 2006 | 1465 | 422 | 1.18 | 4.50 | 0.23 | |||
| 1515 | 115 | 82.60 | ||||||
| 6. Zemirline et al. 2013 | 768 | 30 | 3.33 | 6.66 | ||||
| 7. Henneberg and Henneberg 1999 | 39 | 124 | 13.46 | |||||
| 8. Papp and Porter 1994 | 367 | 104 | 13.46 | |||||
| 617 | 27 | 29.62 | ||||||
| 967 | 77 | 10.38 | ||||||
| 9. Masnicova and Benus 2003 | 1115 | 150 | 2.00 | |||||
| 61 | 3.27 | |||||||
| 65 | 1.47 | |||||||
| 865 | 76 | 3.94 | ||||||
| 38 | 5.26 | |||||||
| 28 | 3.57 | |||||||
| 10. Jankauskas 2001 | 1467 | 633 | 2.21 | 12.95 | ||||
| 11. Jozsa et al. 1992 | 1328 | 233 | 1.71 | 0.42 | ||||
| 12. Groza et al. 2012 | 1660 | 28 | 3.47 | |||||
| 1662 | 129 | 0.77 | ||||||
| 62 | 1.61 | |||||||
| 62 | 1.09 | |||||||
| 1670 | 91 | 1.09 | 1.09 | |||||
| 66 | 1.51 | |||||||
| 13. Kim et al. 2018 | 1666 | 198 | 0.50 | 4.04 | ||||
| 81 | 1.23 | 7.40 | ||||||
| 68 | 1.47 | |||||||
| Publication | Date | Total SS | Male SS | Female SS | S1-S5 | S1 | S1-S2 | S1andS4-S5 |
| 14. Saluja 1988 | 1816 | 112 | 1.78 | 11.60 | 1.78 | |||
| 1928 | 140 | 2.14 | 10.00 | 2.85 | ||||
| 15. Merbs 2004 | 1867 | 218 | 43.11 | |||||
| 16. Solomon et al. 2009 | 1945 | 200 | 10.50 | 8.00 | ||||
| 100 | 17.00 | 13.00 | ||||||
| 100 | 4.00 | 3.00 | ||||||
| 1985 | 200 | 16.50 | 9.50 | |||||
| 100 | 23.00 | 15.00 | ||||||
| 100 | 10.00 | 4.00 | ||||||
| 17. Stewart 1932 | 1900 | 217 | 2.76 | 8.29 | ||||
| 107 | 4.67 | 8.41 | ||||||
| 96 | 1.04 | 9.37 | ||||||
| 18. Avrahami et al. 1994 | 1968 | 273 | 24.24 | |||||
| 137 | 28.46 | |||||||
| 136 | 20.58 | |||||||
| 1958 | 259 | 22.77 | ||||||
| 131 | 27.48 | |||||||
| 128 | 17.96 | |||||||
| 1948 | 248 | 19.35 | ||||||
| 128 | 25.00 | |||||||
| 120 | 13.33 | |||||||
| 1938 | 229 | 8.73 | ||||||
| 111 | 8.10 | |||||||
| 118 | 9.32 | |||||||
| 1928 | 191 | 6.80 | ||||||
| 93 | 7.52 | |||||||
| 98 | 6.12 | |||||||
| Publication | Date | Total SS | Male SS | Female SS | S1-S5 | S1 | S1-S2 | L5-S5 |
| 19. Eubanks and Cheruvu 2009 | 1885 | 2866 | 1.22 | 11.13 | ||||
| 20. Lee et al. 2011 | 1965 | 384 | 2.34 | |||||
| 194 | 2.57 | |||||||
| 190 | 2.10 | |||||||
| 21. Karlin 1935 | 1913 | 75 | 12.00 | 62.00 | 16.00 | 12.00 | ||
| 22. Shore et al. 1932 | 1840 | 78 | 3.84 | |||||
| 23. Sutow and Pryde 1955 | 1945 | 155 | 51.61 | 9.03 | ||||
| 86 | 46.51 | 10.46 | ||||||
| 69 | 57.97 | 7.24 | ||||||
| 1941 | 95 | 44.21 | 6.31 | |||||
| 44 | 47.72 | 9.09 | ||||||
| 51 | 41.17 | 3.92 | ||||||
| 1936 | 108 | 39.81 | 4.62 | |||||
| 48 | 52.08 | 10.41 | ||||||
| 60 | 30.00 | |||||||
| 1921 | 182 | 14.83 | 2.19 | |||||
| 79 | 54.43 | 3.79 | ||||||
| 103 | 20.38 | 0.97 | ||||||
| 1902 | 46 | 23.91 | ||||||
| 1902 | 87 | 16.57 | ||||||
| 1902 | 87 | 22.18 | ||||||
| Publication | Date | Total SS | Male SS | Female SS | S1-S5 | S1 | S1-S2 | L5-S2 |
| 24. Singh 2013 | 1953 | 140 | 3.57 | |||||
| 25. Kubauat et al. 2013 | 1953 | 302 | 10.92 | |||||
| 26. Schweitzer 1992 | 1932 | 177 | 15.81 | |||||
| 32 | 15.62 | |||||||
| 37 | 13.50 | |||||||
| 53 | 16.98 | |||||||
| 56 | 16.07 | |||||||
| 27. Cakiroglu et al. 2014 | 1999 | 233 | 1.28 | |||||
| 151 | 22.31 | 0.85 | ||||||
| 72 | 3.31 | 0.66 | 4.16 | |||||
| 65.27 | 5.55 | |||||||
| 28. Mith and Tayles 2004 | 1994 | 40 | 22.50 | |||||
| 20 | 22.22 | |||||||
| 20 | 27.77 | |||||||
| 29. Piontek 1971 | 1911 | 316 | 1.26 | 3.48 | 3.48 | 1.26 | ||
| 187 | 1.60 | 2.67 | 4.81 | 1.60 | ||||
| 129 | 0.77 | 4.65 | 1.55 | 0.77 | ||||
| 30. 0 et al. 2004 | 1954 | 200 | 4.50 | 3.50 | ||||
| 31. Kumar et al. 2005 | 1997 | 48 | 16.66 | 4.16 | 10.41 | |||
| 32. Kim 2014 | 1996 | 143 | 15.40 | |||||
| 33. Wu et al. 2009 | 1961 | 203 | 2.95 | 18.20 | ||||
| 34. J.W. Wu et al. 2016 | 1954 | 1061 | 11.96 | 0.65 | ||||
| Publication | Date | Total SS | Male SS | Female SS | S1 | L5-S5 | ||
| 35. Fidas et al. 1987 | 1941 | 570 | 28.59 | |||||
| 301 | 37.20 | |||||||
| 269 | 18.95 | |||||||
| 1931 | 877 | 20.98 | ||||||
| 411 | 29.68 | |||||||
| 460 | 13.47 | |||||||
| 1921 | 658 | 20.97 | ||||||
| 333 | 23.72 | |||||||
| 325 | 18.15 | |||||||
| 1911 | 380 | 20.58 | ||||||
| 208 | 25.00 | |||||||
| 172 | 15.11 | |||||||
| 1901 | 173 | 16.76 | ||||||
| 80 | 16.25 | |||||||
| 93 | 17.20 | |||||||
| 1891 | 52 | 19.23 | ||||||
| 24 | 20.83 | |||||||
| 28 | 17.85 | |||||||
| 36. Shin et al. 2013 | 1999 | 160 | 16.25 | |||||
| 37. Urrutia et al. 2016 | 2005 | 228 | 35.08 | |||||
| 38. Al-Dahhan et al, 2020 | 2016 | 180 | 18.5 | 4.40 | ||||
| 39. Albrecht et al. 2017 | 1937 | 53 | 1.25 |
Reference List for Supplementary Materials
Albrecht TL, Scutter SD, Henneberg M. 2007. Radiographical method to access the prevalence of sacral spina bifida occulta. Clin Anat 20:170–174.
Al-Dahhan MH, Mnaather AA, Munshid BA. 2020. Evaluation of spina bifida occulta in young patients presented with lower back pain. Eur J Mol Clin Med 10:4416–4422.
Ali S, Azeemi AA, Shoukat S. 2014. The prevalence of spina bifida occulta in a Pakistani population: a study of dry human sacra. Anaesth, Pain Intensive Care 18:157–161.
Avrahami E, Frishman E, Fridman Z, Azor M. 1994. Spina bifida occulta of S1 is not an innocent finding. Spine 19:12–15.
Cakiroglu B, Tas T, Eyyupoglu SE, Hazar AI, Balci M, Nas Y, Yilmazer F, Aksoy SH. 2014. The adverse influence of spina bifida occulta on the medical treatment outcome of primary monosymptomatic nocturnal enuresis. Archive Italian Urol 86:270–273.
El-Din A, El Banna R. 2006. Congenital anomalies of the vertebral column: a case study on ancient and modern Egypt. Int J Osteoarchaeol 16:200–207.
Eubanks J, Cheruvu VK. 2009. Prevalence of sacral spina bifida occulta and its relationship to age, sex, race, and the sacral table angle. Spine 34:1539–1543.
Fidas A, MacDonald HL, Elton RA, Wild SR, Chrisholm GR, Scott R. 1987. Prevalence and patterns of spina bifida occulta in 2707 normal adults. Clin Rad 38:537–542.
Groza VM, Simalcsik A, Bejenaru L. 2012. Frequency of spina bifida occulta and other occult spinal dysraphism’s in the medieval population of Isas city: skeleton palaeopathology in the necropolis discovered in the eastern part of the Princely Court, 17th century. Biol Anim 58:195–204.
Henneberg RJ, Henneberg M. 1999. Variation in the closure of the sacral canal in the skeletal sample from Pompeii, Italy, 79AD. Perspect Hum Bio 4:177–188.
Hussien FH, El-Din AM, Kandeet W, El Banna R. 2009. Spinal pathological findings in ancient Egyptians of the Greco-Roman period living in Bahriyah Oasis. Int J Osteoarchaeol 19:613–627.
Jankauskas R. 2001. Variations and anomalies of the vertebra column in Lithuanian palaeoosteological samples. Anthropol 39:33–38.
Jozsa L, Pap I, Budapest EF. 1992. The occurrence of spina bifida occulta in medieval and contemporaneous Hungarian populations. Anthropol Hunarica 22:51–60.
Karlin IW. 1953. Incidence of spina bifida occulta in children with and without enuresis. Am J Dis Child 3:374–393.
Kim DW, Lee SJ, Choi EJ, Lee PB, Jo YH, Nahm FS. 2014. Morphological diversities of sacral canal in children: three-dimensional computed tomography study. Korean J Pain 27:253–259.
Kim Y, Kim H, Hong JH, Lee HJ, Kim MJ, Shin DH. 2018. Lumbosacral defects in a 16th–18th century Joseon Dynasty skeletal series from Korea. Biomed Res Int 28:1–8.
Kubauat DM, Nagar SK, Lakhani C. 2013. A study of non-fusion of laminae of the first sacral vertebrae in Western India. Int J Recent Trends Sci Tech 6:122–124.
Kumar P, Aneja S, Kumar R, Taluja V. 2005. Spina bifida occulta in functional enuresis. Indian J Paediatr 72:223–225.
Lee YC, Solomon LB, Ruhli FJ, Schiess R, Ohrstrom L, Sullivan T, Alkadhi H, Henneberg M. 2011. Confirmation of microevolutionary increase of spina bifida occulta among Swiss birth cohorts. Eur Spine J 20:776–780.
Maat GJ, Lonnee HA, Noordhuizen HJ. 1990. Analysis of human skeletons from a Hellenistic period, buried at a ruined Bronze Age building on Failaka, Kuwait. Maison de l’Oreint 18:85–102.
Masnicova S, Benus R. 2003. Developmental anomalies in skeletal remains from the great Moravia and Middle Ages cemeteries at Devin, (Slovakia). Intl J Osteoarchaeol 13:266–274.
Mays S. 2006. Spondylolysis, spondylolisthesis, and lumbo-sacral morphology in a medieval English skeletal population. Am J Phys Anthropol 131:352–362.
McGrath M, Tayles N. 2004. Anatomical observations related to radiological findings in spina bifida -occulta of the lumbo-sacral spine. J Osteopath Med 7:70–78.
Merbs CF. 2004. Sagittal clefting of the body and other vertebral development errors in Canadian Inuit skeletons. Am J Phys Anthropol 123:236–249.
Molto JE, Kirkpatrick CL, Keron J. 2019. The paleoepidemiology of sacral spina bifida occulta in population samples from the Dakhleh Oasis, Egypt. Int J Palaeopathol 26:93–103.
Papp T, Porter RW. 1994. Changes of the lumbar spinal canal proximal to spina bifida occulta. An archaeologic study of clinical significance. Spine 19:1508–1511.
Piontek J. 1971. Variation in the level of closure in the sacral canal of man. Folia Microbiol 4:459–464.
Saluja PG. 1988. The incidence of spina bifida occulta in a historic and a modern London population. J Anat 158:91–93.
Schweitzer ME, Balsam D, Weiss R. 1992. Spina bifida occulta: incidence in parents of offspring with spina bifida cystica. Spine 18:785–786.
Shin SH, Im YJ, Lee MJ, Lee YS, Choi EK, Han SW. 2013. Spina bifida occulta: not to be overlooked in children with nocturnal enuresis. Int J Urol 20:831–835.
Shore LR. 1930. Abnormalities of the vertebral column in a series of skeletons of Bantu natives of South Africa. J Anat 64:206–238.
Singh R. 2013. Classification, causes and clinical implications of sacral spina bifida occulta in Indians. Basic Sci Med. 2:14–20.
Solomon LB, Ruhli FJ, Henneberg M. 2009. Secular trend in the opening of the sacral canal: An Australian study. Spine 34:244–248.
Stewart TD. The vertebral column of the Eskimo. 1932. Am J of Anthropol 17:123–136.
Sutow WW, Pryde AW. 1955. Incidence of spina bifida occulta in relation to age. Am J Dis Child 90:211–217.
Urrutia J, Cuellar J, Zamora T. 2016. Spondylolysis and spina bifida occulta in paediatric patients. Prevalence study using computed tomography as a screening method. Eur Spine J 25:590–595.
Wu JW, Xing YR, Wen YB, Li TF, Xie JF, Feng QD, Shang XP, Li YL, Feng JJ, Wang XX, Zhai RQ, He XF, Chen T, Liu XJ, Wen JG. 2016. Prevalence of spina bifida occulta and its relationship with overactive bladder in middle-aged and elderly Chinese people. Int Neurouro J 20:151–158.
Wu L, Li YK, Li YM, Zhang YQ, Zhong SZ. 2009. Variable morphology of the sacrum in a Chinese population. Clin Anat 22:619–626.
Zemerline A, Vincent JP, Sid-Ahmed S, Nen DL, Dubrana F. 2013. Lumbo-sacral malformations and spina bifida occulta in medieval skeletons from Brittany. Eur J Orthop Surg Traumatol 23:149–153.


Received: 25.04.2022; Revised: 04.05.2022; Accepted: 05.05.2022