The Association of Body Temperature with Longevity: Insights from Historical Cohorts
Piotr Paweł Chmielewski
 https://orcid.org/0000-0002-6995-123X
https://orcid.org/0000-0002-6995-123X
Division of Anatomy, Department of Human Morphology and Embryology, Faculty of Medicine, Wroclaw Medical University, Wroclaw, Poland
Krzysztof Chmielowiec
 https://orcid.org/0000-0003-4254-5466
https://orcid.org/0000-0003-4254-5466
Department of Hygiene and Epidemiology, Collegium Medicum, The University of Zielona Gora, Zielona Gora, Poland
Abstract. Effective thermoregulation is crucial for maintaining homeostasis. Previous research has suggested a link between lower steady-state body temperature and longevity, particularly in physically healthy, non-obese older adults. However, the exact mechanisms behind this relationship remain unclear. Despite the physiological insights gained from studies on body temperature, limited attention has been given to its potential role as a biomarker of longevity in physically healthy older populations. This study aimed to evaluate the relationship between body temperature and longevity using historical data from two cohorts. The longitudinal cohort consisted of 142 individuals, followed for 25 years beginning at age 45, while the cross-sectional cohort included 204 individuals stratified into four lifespan categories. To examine age-related trends in body temperature, Page’s test was employed, and ordinal regression was used. The analysis revealed a significant decrease in body temperature in women with age, while men showed no significant change. The cross-sectional analysis indicated a trend toward lower body temperatures in individuals with longer lifespans. Lower body temperature may reflect a reduced metabolic rate, thereby mitigating oxidative stress and molecular damage, both of which are known to drive aging and limit lifespan. Furthermore, lower body temperatures may signal a favorable inflammatory profile, which could translate into slower aging and increased survival. However, the observed sex-specific differences in thermoregulatory patterns raise important questions about the role of hormonal influences, such as estrogen levels. Overall, these findings suggest that lower lifetime steady-state body temperature may be a biomarker of healthy aging and longevity, warranting further exploration of its mechanistic underpinnings.
Key words: age, aging, biomarker, body temperature, lifespan, longevity, survival
Introduction
Identifying reliable biomarkers of healthy aging and longevity is one of the central challenges in biogerontology and medical research (Martin-Ruiz et al. 2011; Dodds et al. 2014; Arai et al. 2015; Sayer and Kirkwood 2015; Chen et al. 2016; Davis et al. 2016; Ferrucci et al. 2018; Levine et al. 2018; Smith et al. 2019; Guerville et al. 2020; He et al. 2024). Among the various candidates, core body temperature stands out as an intriguing and potentially informative biomarker (Conti 2008; Lehmann et al. 2013; Keil et al. 2015), as studies have associated lower temperatures with longer lifespan and higher temperatures with shorter lifespan in diverse species, including animal models of aging (e.g., Caenorhabditis elegans, Drosophila melanogaster, and mice) as well as humans (Rikke and Johnson 2004; Waalen and Buxbaum 2011; Palani et al. 2023 Chmielewski et al. 2025). Reflecting the delicate equilibrium between heat production and dissipation, body temperature not only underpins homeostatic control but also encapsulates the cumulative effects of metabolic, immunological, and environmental influences on aging organisms (Roth et al. 2002; Ruggiero et al. 2008; Åström et al. 2011; Soare et al. 2011; Keil et al. 2015; Geneva et al. 2019; Lee et al. 2023; Kowald et al. 2024; Li et al. 2024).
In healthy individuals, body temperature follows a circadian rhythm, typically reaching its lowest point in the early morning and peaking in the late afternoon. Such diurnal fluctuations underscore the importance of considering the timing of temperature measurements, as sporadic readings may fail to capture the basal set point that is critical for assessing long-term health and survival (Simonsick et al. 2016). The distinction between adaptive and maladaptive alterations in body temperature is further highlighted by the differential responses seen in hyperthermia versus fever. Hyperthermia is characterized by an excessive accumulation of heat that overwhelms the body’s dissipative mechanisms, which is harmful to health. In contrast, fever is a regulated increase in the body’s temperature set point, which is orchestrated by endogenous pyrogens such as interleukins (e.g., IL-1, IL-6, and IL-8), interferons (e.g., interferon-γ), tumor necrosis factor-β etc., in response to infectious or inflammatory stimuli. The fever response represents an adaptive strategy that evolved to combat pathogens and increase survival.
Previous studies have suggested that lower basal body temperature may be a biomarker of healthy aging and greater longevity, particularly in physically healthy, non-obese older adults (Waalen and Buxbaum 2011; Simonsick et al. 2016; Chmielewski et al. 2025). However, this association remains understudied in the Polish population, and it is unclear whether reduced core temperature directly influences longevity or simply serves as a surrogate marker for other health-promoting processes. Enhanced immune responses, decreased chronic low-grade systemic inflammation (CLSI), and the absence of disease or infirmity may all contribute to a reduced temperature profile, which could also correlate with longevity benefits in the elderly population (Franceschi and Campisi 2014; Nilsson et al. 2014; Proctor et al. 2015; Chmielewski et al. 2016; Chmielewski and Strzelec 2018; Ferrucci and Fabbri 2018).
One should consider whether there are factors and mechanisms that underlie the association between lower lifetime steady-state body temperature and extended longevity, and, if so, identify what they are. For instance, the phenomenon of lower body temperature has been closely linked with caloric restriction (CR), which is a well-established intervention that promotes longevity across a range of species (Colman et al. 2009; Fontana et al. 2010; Anderson and Weindruch 2010; Chmielewski 2017; 2020; Picca et al. 2017; Campisi et al. 2019; Dorling et al. 2020; Speakman 2020; Giacomello and Toniolo 2021; Hoong and Chua 2021; Sultanova et al. 2021; Waziry et al. 2023; Di Francesco et al. 2024; Greenhill 2024). CR is known to induce a metabolic shift characterized by reduced energy expenditure and improved physiological efficiency, which is often accompanied by a modest decline in core temperature (Carrillo and Flouris 2011).
Furthermore, subclinical conditions such as endocrine disorders, latent infections (e.g., tuberculosis, hepatitis B and C, and HIV), autoimmune disorders (e.g., lupus), as well as insulin resistance, metabolic dysregulation, and type 2 diabetes mellitus, have been linked to elevated body temperature and reduced survival. Moreover, unhealthy lifestyle factors, including chronic psychological stress, long-term alcohol consumption, and inadequate sleep, can lead to changes in inflammatory cytokines and white blood cell counts (Mullington et al. 2010; Knutson 2012; Chen et al. 2024). Conversely, progressive sarcopenia and atherosclerosis—conditions commonly observed in older adults—can lead to a decline in body temperature, but they are also associated with increased cardiovascular risk and premature mortality (Barquera et al. 2015; Herrington et al. 2016; Agnelli et al. 2020; Bayraktar et al. 2020; He et al. 2021).
Despite the physiological insights gained from studies on body temperature (Lu et al. 2010; Obermeyer et al. 2017; Diamond et al. 2021), little attention has been devoted to its potential role as an independent biomarker of longevity in physically healthy older populations. Most clinical measurements of body temperature are conducted during acute illness or hospitalization, which restricts our understanding of its normative patterns in the context of longevity among community-dwelling older adults. This gap is especially pronounced in historical cohorts, where comprehensive longitudinal data are extraordinarily scarce. Consequently, key questions regarding the typical profiles of core body temperature and their association with reliable markers of survival (e.g., inflammatory biomarkers and epigenetic ‘clocks’) in long-lived versus short-lived individuals remain largely unexplored.
This study aims to address this gap by analyzing both longitudinal and cross-sectional data to investigate whether lower body temperature is associated with greater longevity in physically healthy older adults within the Polish population.
Materials and methods
Study Population
The study adhered to the principles of the Declaration of Helsinki. Archival clinical data from physical examinations at the Mental Health Center in the vicinity of Zielona Góra, Lubuskie Province, Poland, were used for this research. Ethical approval for the study was granted by the institutional review board in 2007 as part of a doctoral research project. All medical records were anonymized to protect patient confidentiality and subsequently used to construct a comprehensive database incorporating both longitudinal and cross-sectional data.
The longitudinal cohort comprised 142 residents (68 men and 74 women), who were monitored continuously from ages 45 to 70 years. These individuals reached the age of 70 years, after which their outcomes were not further tracked. The cross-sectional cohort consisted of 204 individuals, including 98 men and 106 women, who were assessed during periodic clinical examinations at multiple intervals. These participants were stratified into four lifespan categories based on death certificates: (1) short lifespan: 15 men (aged 50–58 years, mean age 53 years) and 12 women (aged 50–58 years, mean age 53 years), (2) medium lifespan: 26 men (aged 58–65 years, mean age 63 years) and 30 women (aged 58–65 years, mean age 63 years), (3) long lifespan: 42 men (aged 65–72 years, mean age 68 years) and 40 women (aged 65–72 years, mean age 68 years), and (4) very long lifespan: 15 men and 24 women (aged 76+). The short lifespan category included only individuals who lived significantly below their life expectancy at birth (< e0), while the medium and long lifespan categories contained individuals with life expectancies close to e0. The very long lifespan category exclusively included individuals who surpassed 76 years, thus exceeding the e0 threshold.
Physiological Measurements
Sublingual body temperature (°C) was measured monthly under clinical conditions using a standard thermometer with 0.1°C accuracy. All measurements were taken systematically by trained medical personnel in standardized conditions at the same medical institution, typically in the morning. This study used only averaged data derived from 60 measurements per 5-year period for each individual in the longitudinal cohort, resulting in 300 measurements per person over the entire study period.
In the cross-sectional cohort, each individual contributed at least several dozens of measurements. These rigorous data collection practices ensured statistical robustness. Comprehensive details regarding the study cohorts, including the daily routines of patients and medical staff, as well as the data collection procedures, have been documented in previous publications (Borysławski et al. 2015; Chmielewski et al. 2015; 2016; 2017; 2025).
Statistical Analysis
To calculate reliable estimates of central tendency and variability, we aggregated frequently repeated measurements for each participant, including arithmetic means, medians, percentiles, and standard deviations (SDs). This approach minimized variability and enhanced the reliability of the findings. The normality of data distribution was tested with the Shapiro-Wilk test (Shapiro and Wilk 1965). The significance level was set at 0.05.
To examine whether a trend exists in body temperature with age, Page’s test (Page 1963) was employed. This test serves as an alternative to Friedman’s test and has greater statistical power. The null hypothesis in Page’s test, similar to Friedman’s test, assumes equality among the measures of central tendency across all analyzed groups. However, the alternative hypothesis in Page’s test differs from that in Friedman’s test. It posits that for the measures of central tendency in n studied groups—θ1, θ2, θ3, …, θn —the following sequence of inequalities holds: θ1 ≤ θ2 ≤ θ3 ≤ … ≤ θn, with at least one strict inequality. This implies the presence of an increasing trend in the measures of central tendency. In the present analysis, this would correspond to an increase in the median values of the studied variables across successive age groups: 45, 50, 55, 60, 65, and 70 years.
Ordinal regression was conducted using the Cumulative Link Model (CLM), which accounts for covariates and provides a robust framework for modeling ordinal outcomes. All statistical analyses were performed using R software (R Foundation for Statistical Computing, Vienna, Austria).
Results
Longitudinal Cohort
The normality of data distribution was confirmed by the Shapiro-Wilk test (p > 0.05). In men, no significant change in body temperature was observed over the study period (Table 1, Fig. 1), as Page’s test did not reveal any significant increasing or decreasing trend in body temperature for men (test statistic = 4965.5; p = 0.692). In contrast, for women, Page’s test identified as significant decreasing trend (test statistic = 5876; p < 0.05), indicating a significant decline in body temperature associated with aging (Table 2, Fig. 1).
| Age | Men | |||||
|---|---|---|---|---|---|---|
| Min | Q1 | Median | Q3 | Max | Mean ± SD | |
| 45 | 36.0 | 36.4 | 36.6 | 36.7 | 37.2 | 36.6 ± 0.2 |
| 50 | 36.2 | 36.5 | 36.6 | 36.6 | 37.0 | 36.6 ± 0.2 |
| 55 | 36.0 | 36.5 | 36.6 | 36.7 | 37.0 | 36.6 ± 0.2 |
| 60 | 36.3 | 36.5 | 36.6 | 36.7 | 37.0 | 36.6 ± 0.2 |
| 65 | 36.2 | 36.5 | 36.6 | 36.7 | 36.9 | 36.6 ± 0.2 |
| 70 | 36.0 | 36.4 | 36.6 | 36.7 | 37.2 | 36.6 ± 0.1 |
| Age | Women | |||||
|---|---|---|---|---|---|---|
| Min | Q1 | Median | Q3 | Max | Mean ± SD | |
| 45 | 35.8 | 36.4 | 36.5 | 36.6 | 37.0 | 36.5 ± 0.3 |
| 50 | 36.0 | 36.4 | 36.5 | 36.6 | 36.9 | 36.5 ± 0.2 |
| 55 | 36.0 | 36.5 | 36.6 | 36.6 | 36.9 | 36.6 ± 0.2 |
| 60 | 36.2 | 36.5 | 36.6 | 36.6 | 36.9 | 36.6 ± 0.2 |
| 65 | 36.2 | 36.5 | 36.6 | 36.7 | 37.0 | 36.6 ± 0.2 |
| 70 | 36.2 | 36.5 | 36.6 | 36.8 | 37.1 | 36.6 ± 0.2 |
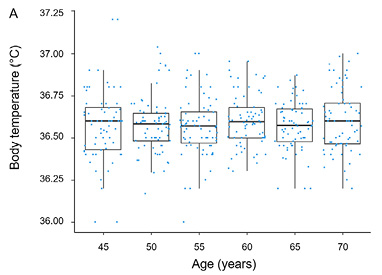
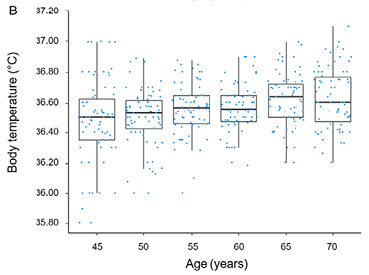
Cross-Sectional Cohort
The basic descriptive statistics for men and women in the cross-sectional cohort are summarized in Tables 3 and 4, respectively. Age-related changes in measures of central tendency, along with standard deviations across consecutive lifespan categories, are presented in Fig. 2. The cross-sectional analysis revealed a trend toward lower body temperatures in long-live men and women, but it was statistically non-significant (p > 0.05). The results of the CLM analysis for men and women are provided in Table 5.
| Lifespan category | Men | |||||
|---|---|---|---|---|---|---|
| Min | Q1 | Median | Q3 | Max | Mean ± SD | |
| Short | 36.3 | 36.4 | 36.7 | 36.8 | 36.9 | 36.6 ± 0.2 |
| Medium | 36.0 | 36.5 | 36.6 | 36.6 | 36.8 | 36.5 ± 0.2 |
| Long | 36.2 | 36.5 | 36.5 | 36.6 | 37.0 | 36.5 ± 0.2 |
| Very long | 36.0 | 36.4 | 36.6 | 36.6 | 36.7 | 36.5 ± 0.2 |
| Lifespan category | Women | |||||
|---|---|---|---|---|---|---|
| Min | Q1 | Median | Q3 | Max | Mean ± SD | |
| Short | 36.0 | 36.5 | 36.6 | 36.8 | 36.9 | 36.6 ± 0.2 |
| Medium | 36.3 | 36.5 | 36.6 | 36.7 | 37.0 | 36.6 ± 0.2 |
| Long | 36.0 | 36.5 | 36.5 | 36.6 | 37.0 | 36.5 ± 0.2 |
| Very long | 36.0 | 36.5 | 36.6 | 36.7 | 36.8 | 36.5 ± 0.2 |
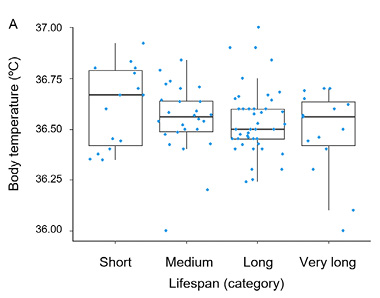
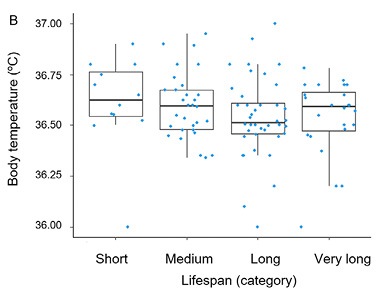
| Sex | Estimate | Standard Error | z-value | Pr (>|z|) | Odds Ratio | 2.5% | 97.5% |
|---|---|---|---|---|---|---|---|
| Men | –1.892 | 1.086 | –1.742 | 0.0815 | 0.1508 | 0.0172 | 1.236 |
| Women | –1.486 | 0.948 | –1.568 | 0.1169 | 0.2262 | 0.0344 | 1.442 |
Discussion
This study builds on previous research investigating the relationship between resting body temperature and longevity (Chmielewski et al. 2015; 2025) by analyzing historical data from long-term residents of the same mental health center. The findings offer novel insights into the association between body temperature and long-term survival. Specifically, the analysis revealed sex-specific differences in long-term trends, warranting further investigation into the link between lower body temperature and increased longevity.
The longitudinal analysis showed that body temperature declined with advancing age in women, while no significant age-related trend was observed in men. Similarly, the cross-sectional data, which categorized individuals by lifespan, revealed a downward trend, with older individuals tending to have lower body temperatures compared to those with shorter lifespans. Although this difference did not reach statistical significance, it suggests a potential trend worthy of further exploration. For instance, it was claimed that because women generally have a higher body temperature than men—and yet consistently outlive them—it is unlikely that core body temperature affects longevity (see Introduction). However, studies have shown that women have only a slightly higher body temperature than men (approximately 0.5 °C, largely attributable to temperature fluctuations during the menstrual cycle, which diminish after menopause), and our analysis clearly demonstrated that during the study period—between the ages of 45 and 70—a statistically significant reduction in body temperature occurred in women but not in men. Thus, since only women experienced a significant reduction in body temperature while living longer, the notion that core body temperature does not affect human longevity becomes less tenable.
This serves as an example of the challenges in redefining classical models and views on aging in light of emerging experimental evidence (Chmielewski 2017; 2020). One classical theory on the evolution of aging is the Disposable Soma Theory of Aging (DSTA), formulated by Thomas Kirkwood (1977), which posits that aging evolved as a byproduct of natural selection due to an evolutionary trade-off between resources allocated to somatic maintenance and sexual reproduction—that is, the more an organism invests in sexual reproduction, the less is available for somatic maintenance, and vice versa (Kirkwood and Holliday 1979; Kirkwood and Rose 1991; Drenos and Kirkwood 2005). This influential, mathematically rigorous, and elegant theory holds that our bodies can be considered as disposable ‘containers’ for our genes and that, beyond an ‘essential lifespan’ (roughly between 35 and 45 years), they begin to deteriorate because evolution did not expect them to function indefinitely or much longer than this critical period, e.g., due to the selection shadow (Chmielewski 2017; 2019).
Although alternative models have been proposed (Maklakov and Chapman 2019; Speakman 2020; Carlsson et al. 2021; Gems 2022; Lemaître et al. 2024; Mitchell et al. 2024), including markedly different perspectives (Longo et al. 2005; Longo and Anderson 2022), the DSTA remains one of the more robust and influential frameworks in current biogerontology (Jasienska 2009; Hammers et al. 2013; Ziomkiewicz et al. 2016; Jasienska et al. 2017; Collins et al. 2023). Indeed, the DSTA can help elucidate our findings: despite investing more in sexual reproduction, women still live longer than men. Furthermore, the higher core body temperature that women experience during their fertile period (e.g., due to hormonal changes during the menstrual cycle) may represent one example of the biological costs of reproduction that women incur. Taken together, these findings suggest that lower lifetime steady-state body temperature may be associated with increased longevity. This finding is in agreement with previous studies (Rikke and Johnson 2004; Waalen and Buxbaum 2011; Simonsick et al. 2016; Palani et al. 2023 Chmielewski et al. 2025).
We hypothesize that a lower body temperature may reflect a reduced basal metabolic rate (BMR), which is a condition that has been associated with decreased production of reactive oxygen species (ROS) and a consequent reduction in cumulative molecular and cellular damage. In animal models, lower metabolic rates have been correlated with extended lifespans, positing that slower metabolic rates may help to mitigate the deleterious effects of oxidative stress. Additionally, the possibility exists that lower body temperature is indicative not only of reduced metabolic activity but also of a more favorable inflammatory profile, as elevated body temperature may signal the presence of chronic systemic inflammation, subclinical diseases, latent infections, or autoimmune processes—conditions that are known to contribute to age-related morbidity and mortality (Chmielewski 2018; Chmielewski and Strzelec 2018). Our observation that short-lived individuals tend to have higher temperatures suggests that elevated body temperature could indicate an underlying, suboptimal inflammatory state. This may predispose aging individuals to earlier mortality. Conversely, a lower body temperature in long-lived individuals could denote a more robust immune system or an absence of deleterious inflammatory activity, thereby supporting longevity.
The sex-specific divergence observed in our study enriches the discussion. Women not only displayed a significant decline in body temperature with advancing age, but they also, as other studies have shown, tend to have slightly higher baseline temperatures than men, yet women consistently outlive men (McGann et al. 1993; Chmielewski 2015; 2016; 2022; 2024; Keil et al. 2015; Chmielewski and Borysławski 2016; Baum et al. 2021; Öngel et al. 2021; Chmielewski et al. 2023). The dichotomy between the temperature trends observed in men and women raises intriguing questions about the underlying physiological mechanisms at play. It is possible that hormonal differences, variations in body composition, or disparities in the prevalence of autoimmune conditions contribute to these sex-specific patterns. For instance, the higher propensity for autoimmune disorders among women might initially elevate body temperature (Dolgin 2024). However, as adaptive mechanisms evolve, a subsequent decline might reflect a rebalancing that ultimately favors longevity. In contrast, the absence of a similar trend in men could indicate that other compensatory mechanisms, such as differences in metabolic regulation or thermogenic responses, come into play.
The interplay between body mass index (BMI), systemic inflammation, and body temperature should not be overlooked. Prior research has documented a positive association between higher BMI and increased body temperature, as well as between elevated temperature and higher mortality rates (Waalen and Buxbaum 2011; Simonsick et al. 2016; Chmielewski et al. 2025). It is plausible that individuals with a lower BMI, who may also experience reduced systemic inflammation, are more likely to exhibit a lower steady-state temperature and, consequently, a longer lifespan. This hypothesis is further bolstered by the observation that higher white blood cell counts—often reflective of ongoing inflammatory processes—are associated with poorer health outcomes in older adults (Ruggiero et al. 2007; Nilsson et al. 2014; Chmielewski 2018; Chmielewski et al. 2016; Chmielewski and Strzelec 2018). The converging lines of evidence thus suggest that a low basal temperature might be more than a passive marker of metabolic rate; it could also be a surrogate for an overall anti-inflammatory state that is conducive to healthy aging.
Notwithstanding the implications of these findings, several limitations must be acknowledged. First, the reliance on historical data from a specific institutionalized population raises questions about the generalizability of the results to the broader aging population, as clinical data may be affected by confounding variables (Chmielewski et al. 2015; 2016; 2025). Second, the cross-sectional component, while suggestive of a relationship between temperature and longevity, is inherently limited by its observational nature and the potential for confounding variables—such as undiagnosed subclinical conditions or lifestyle factors—that may not have been fully accounted for.
Despite these constraints, our study contributes to a growing body of literature that challenges traditional interpretations of age-related thermoregulatory decline. Rather than viewing lower body temperature simply as a result of diminished thermoregulatory function in the elderly, our findings raise the possibility that a lower steady-state temperature may be an adaptive trait reflecting a finely tuned balance between metabolic efficiency, immune function, and systemic inflammation. This interpretation suggests that effective interventions targeting the underlying mechanisms of aging could one day offer novel strategies for promoting longevity (Chmielewski et al. 2024; Li et al. 2024; Mahoney et al. 2024).
Future investigations should aim to clarify these relationships by employing prospective, population-based designs with rigorous standardization of temperature measurements. Such studies would benefit from the inclusion of a comprehensive set of biomarkers, including detailed assessments of metabolic rate, inflammatory mediators, and immune function, in order to disentangle the complex interdependencies underlying the observed associations. Furthermore, exploring the molecular and genetic determinants of thermoregulation across different populations could provide insight into why some individuals exhibit lower baseline temperatures and enjoy a survival advantage.
In conclusion, this study offers preliminary evidence suggesting that lower lifetime steady-state body temperature could be a biomarker of longevity. The observed trends, suggesting that long-lived individuals tend to have lower body temperature, support the hypothesis that a lower metabolic rate and reduced systemic inflammation are beneficial for survival. However, the sex-specific differences and the lack of statistically significant differences between lifespan categories caution against oversimplification and highlight the complexity of the relationship between body temperature and longevity. Our findings also emphasize the need for further research to clarify the causal pathways and potential clinical implications of these associations.
References
Agnelli G, Belch JJF, Baumgartner I, Giovas P, Hoffmann U. 2020. Morbidity and mortality associated with atherosclerotic peripheral artery disease: A systematic review. Atherosclerosis 293:94–100. https://doi.org/10.1016/j.atherosclerosis.2019.09.012
Anderson RM, Weindruch R. 2010. Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol Metab 21:134–41. https://doi.org/10.1016/j.tem.2009.11.005
Arai Y, Martin-Ruiz CM, Takayama M, Abe Y, Takebayashi T, Koyasu S, Suematsu M, Hirose N, von Zglinicki T. 2015. Inflammation, but not telomere length, predicts successful ageing at extreme old age: A longitudinal study of semi-supercentenarians. EBioMedicine 2:1549–58. https://doi.org/10.1016/j.ebiom.2015.07.029
Åström DO, Forsberg B, Rocklöv J. 2011. Heat wave impact on morbidity and mortality in the elderly population: A review of recent studies. Maturitas 69:99–105. https://doi.org/10.1016/j.maturitas.2011.03.008
Barquera S, Pedroza-Tobías A, Medina C, Hernández-Barrera L, Bibbins-Domingo K, Lozano R, Moran AE. 2015. Global Overview of the Epidemiology of Atherosclerotic Cardiovascular Disease. Arch Med Res 46:328–38. https://doi.org/10.1016/j.arcmed.2015.06.006
Baum F, Musolino C, Gesesew HA, Popay J. 2021. New Perspective on Why Women Live Longer Than Men: An Exploration of Power, Gender, Social Determinants, and Capitals. Int J Environ Res Public Health 18:661. https://doi.org/10.3390/ijerph18020661
Bayraktar E, Tasar PT, Binici DN, Karasahin O, Timur O, Sahin S. 2020. Relationship between Sarcopenia and Mortality in Elderly Inpatients. Eurasian J Med 52:29–33. https://doi.org/10.5152/eurasianjmed.2020.19214
Borysławski K, Chmielowiec K, Chmielewski P, Chmielowiec J. 2015. Zmiany z wiekiem wybranych cech antropometrycznych, fizjologicznych i biochemicznych oraz ich związek z długością życia mężczyzn i kobiet. Monographs of Physical Anthropology 2:1–224. Available at: http://antropologia.upwr.edu.pl/wp-content/uploads/mpa_vol2_2015.pdf [Accessed 11 March 2025].
Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E. 2019. From discoveries in ageing research to therapeutics for healthy ageing. Nature 571:183–92. https://doi.org/10.1038/s41586-019-1365-2
Carlsson H, Ivimey-Cook E, Duxbury EML, Edden N, Sales K, Maklakov AA. 2021. Ageing as “early-life inertia”: Disentangling life-history trade-offs along a lifetime of an individual. Evol Lett 5:551–64. https://doi.org/10.1002/evl3.254
Carrillo AE, Flouris AD. 2011. Caloric restriction and longevity: effects of reduced body temperature. Ageing Res Rev 10:153–62. https://doi.org/10.1016/j.arr.2010.10.001
Chen BH, Marioni RE, Colicino E, Peters MJ, Ward-Caviness CK, Tsai PC, Roetker NS, Just AC, Demerath EW, Guan W, Bressler J, Fornage M, Studenski S, Vandiver AR, Moore AZ, Tanaka T, Kiel DP, Liang L, Vokonas P, Schwartz J, Lunetta KL, Murabito JM, Bandinelli S, Hernandez DG, Melzer D, Nalls M, Pilling LC, Price TR, Singleton AB, Gieger C, Holle R, Kretschmer A, Kronenberg F, Kunze S, Linseisen J, Meisinger C, Rathmann W, Waldenberger M, Visscher PM, Shah S, Wray NR, McRae AF, Franco OH, Hofman A, Uitterlinden AG, Absher D, Assimes T, Levine ME, Lu AT, Tsao PS, Hou L, Manson JE, Carty CL, LaCroix AZ, Reiner AP, Spector TD, Feinberg AP, Levy D, Baccarelli A, van Meurs J, Bell JT, Peters A, Deary IJ, Pankow JS, Ferrucci L, Horvath S. 2016. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging 8:1844–65. https://doi.org/10.18632/aging.101020
Chen Z, Ding C, Chen K, Lu C, Li Q. 2024. Exploring the impact of inflammatory cytokines on alcoholic liver disease: a Mendelian randomization study with bioinformatics insights into potential biological mechanisms. Am J Drug Alcohol Abuse 50:643–58. https://doi.org/10.1080/00952990.2024.2402569
Chmielewski P. 2015. Wysokość ciała i miesiąc urodzenia a długość życia osób zmarłych w Polsce w latach 2004–2008. [pdf] Doctoral dissertation. Available at: https://ruj.uj.edu.pl/server/api/core/bitstreams/1ecd8760-57c6-4e5c-8f48-7f5a7498b52c/content [Accessed 11 March 2025].
Chmielewski P. 2016. The relationship between adult stature and longevity: tall men are unlikely to outlive their short peers – evidence from a study of all adult deaths in Poland in the years 2004–2008. Anthropol Rev 79:439–60. https://doi.org/10.1515/anre-2016-0032
Chmielewski P. 2017. Rethinking modern theories of ageing and their classification: the proximate mechanisms and the ultimate explanations. Anthropol Rev 80:259–72. https://doi.org/10.1515/anre-2017-0021
Chmielewski P. 2018. Leukocyte count, systemic inflammation, and health status in older adults: a narrative review. Anthropol Rev 81:81–101. https://doi.org/10.2478/anre-2018-0007
Chmielewski PP. 2019. Human ageing, longevity and evolution: can ageing be programmed? Anthropol Rev 82:417–33. https://doi.org/10.2478/anre-2019-0032
Chmielewski PP. 2020. Human ageing as a dynamic, emergent and malleable process: from disease-oriented to health-oriented approaches. Biogerontology 21:125–30. https://doi.org/10.1007/s10522-019-09839-w
Chmielewski PP. 2022. Do taller people live longer? Evaluating the relationship between adult stature and longevity. Med J Cell Biol 10:176–83. https://doi.org/10.2478/acb-2022-0025
Chmielewski PP. 2024. The association between body height and longevity: evidence from a national population sample. Folia Morphol 83:139–45. https://doi.org/10.5603/FM.a2023.0005
Chmielewski P, Borysławski K, Chmielowiec K, Chmielowiec J. 2015. Longitudinal and cross-sectional changes with age in selected anthropometric and physiological traits in hospitalized adults: and insight from the Polish Longitudinal Study of Aging (PLSA). Anthropol Rev 78:317–36. https://doi.org/10.1515/anre-2015-0025
Chmielewski P, Borysławski K. 2016. Understanding the links between month of birth, body height, and longevity: why some studies reveal that shorter people live longer – further evidence of seasonal programming from the Polish population. Anthropol Rev 79:375–95. https://doi.org/10.1515/anre-2016-0028
Chmielewski PP, Borysławski K, Chmielowiec K, Chmielowiec J, Strzelec B. 2016. The association between total leukocyte count and longevity: Evidence from longitudinal and cross-sectional data. Ann Anat 204:1–10. https://doi.org/10.1016/j.aanat.2015.09.002
Chmielewski P, Strzelec B, Chmielowiec J, Chmielowiec K, Borysławski K. 2017. Association of serum bilirubin with longevity: Evidence from a retrospective longitudinal study and cross-sectional data. Anthropol Rev 80:335–48. https://doi.org/10.1515/anre-2017-0024
Chmielewski PP, Strzelec B. 2018. Elevated leukocyte count as a harbinger of systemic inflammation, disease progression, and poor prognosis: a review. Folia Morphol 77:171–8. https://doi.org/10.5603/FM.a2017.0101
Chmielewski PP, Kozieł S, Borysławski K. 2023. Do the short die young? Evidence from a large sample of deceased Polish adults. Anthropol Rev 86:77–90. https://doi.org/10.18778/1898-6773.86.1.07
Chmielewski PP, Data K, Strzelec B, Farzaneh M, Anbiyaiee A, Zaheer U, Uddin S, Sheykhi-Sabzehpoush M, Mozdziak P, Zabel M, Dzięgiel P, Kempisty B. 2024. Human Aging and Age-Related Diseases: From Underlying Mechanisms to Pro-Longevity Interventions. Aging Dis 16:1–25. https://doi.org/10.14336/AD.2024.0280
Chmielewski PP, Strzelec B, Data K, Chmielowiec K, Mozdziak P, Kempisty B. 2025. Resting Body Temperature and Long-Term Survival in Older Adults at a Mental Health Center: Cross-Sectional and Longitudinal Data. J Clin Med. 14:713. https://doi.org/10.3390/jcm14030713
Collins DH, Prince DC, Donelan JL, Chapman T, Bourke AFG. 2023. Costs of reproduction are present but latent in eusocial bumblebee queens. BMC Biol 21:153. https://doi.org/10.1186/s12915-023-01648-5
Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. 2009. Calorie restriction delays disease onset and mortality in rhesus monkeys. Science 325:201–04. https://doi.org/10.1126/science.1173635
Conti B. 2008. Considerations on temperature, longevity and aging. Cell Mol Life Sci 65:1626–30. https://doi.org/10.1007/s00018-008-7536-1
Davis JL, Moutinho V Jr, Panageas KS, Coit DG. 2016. A peripheral blood biomarker estimates probability of survival: the neutrophil–lymphocyte ratio in noncancer patients. Biomarkers in Medicine 10:953–57. https://doi.org/10.2217/bmm-2016-0103
Di Francesco A, Deighan AG, Litichevskiy L, Chen Z, Luciano A, Robinson L, Garland G, Donato H, Vincent M, Schott W, Wright KM, Raj A, Prateek GV, Mullis M, Hill WG, Zeidel ML, Peters LL, Harding F, Botstein D, Korstanje R, Thaiss CA, Freund A, Churchill GA. 2024. Dietary restriction impacts health and lifespan of genetically diverse mice. Nature 634:684–92. https://doi.org/10.1038/s41586-024-08026-3
Diamond A, Lye CT, Prasad D, Abbott D. 2021. One size does not fit all: Assuming the same normal body temperature for everyone is not justified. PLoS One 16:e0245257. https://doi.org/10.1371/journal.pone.0245257
Dodds RM, Syddall HE, Cooper R, Benzeval M, Deary IJ, Dennison EM, Der G, Gale CR, Inskip HM, Jagger C, Kirkwood TB, Lawlor DA, Robinson SM, Starr JM, Steptoe A, Tilling K, Kuh D, Cooper C, Sayer AA. 2014. Grip strength across the life course: normative data from twelve British studies. PLoS One 9:e113637. https://doi.org/10.1371/journal.pone.0113637
Dolgin E. 2024. Why autoimmune disease is more common in women: X chromosome holds clues. Nature 626:466. https://doi.org/10.1038/d41586-024-00267-6
Dorling JL, Martin CK, Redman LM. 2020. Calorie restriction for enhanced longevity: The role of novel dietary strategies in the present obesogenic environment. Ageing Res Rev 64:101038. https://doi.org/10.1016/j.arr.2020.101038
Drenos F, Kirkwood TB. 2005. Modelling the disposable soma theory of ageing. Mech Ageing Dev 126:99–103. https://doi.org/10.1016/j.mad.2004.09.026
Ferrucci L, Fabbri E. 2018. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 15:505–22. https://doi.org/10.1038/s41569-018-0064-2
Ferrucci L, Levine ME, Kuo PL, Simonsick EM. 2018. Time and the Metrics of Aging. Circ Res 123:740–4. https://doi.org/10.1161/CIRCRESAHA.118.312816
Fontana L, Partridge L, Longo VD. 2010. Extending healthy life span-from yeast to humans. Science 328:321–6. https://doi.org/10.1126/science.1172539
Franceschi C, Campisi J. 2014. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 69:S4–9. https://doi.org/10.1093/gerona/glu057
Gems D. 2022. The hyperfunction theory: An emerging paradigm for the biology of aging. Ageing Res Rev 74:101557. https://doi.org/10.1016/j.arr.2021.101557
Geneva II, Cuzzo B, Fazili T, Javaid W. 2019. Normal Body Temperature: A Systematic Review. Open Forum Infect Dis 6:ofz032. https://doi.org/10.1093/ofid/ofz032
Giacomello E, Toniolo L. 2021. The Potential of Calorie Restriction and Calorie Restriction Mimetics in Delaying Aging: Focus on Experimental Models. Nutrients 13:2346.
Greenhill C. 2024. The complex effects of dietary restriction on longevity and health. Nat Rev Endocrinol 20:697. https://doi.org/10.3390/nu13072346
Guerville F, De Souto Barreto P, Ader I, Andrieu S, Casteilla L, Dray C, Fazilleau N, Guyonnet S, Langin D, Liblau R, Parini A, Valet P, Vergnolle N, Rolland Y, Vellas B. 2020. Revisiting the Hallmarks of Aging to Identify Markers of Biological Age. J Prev Alzheimers Dis 7:56–64. https://doi.org/10.14283/jpad.2019.50
Hammers M, Richardson DS, Burke T, Komdeur J. 2013. The impact of reproductive investment and early-life environmental conditions on senescence: support for the disposable soma hypothesis. J Evol Biol 26:1999–2007. https://doi.org/10.1111/jeb.12204
He N, Zhang Y, Zhang L, Zhang S, Ye H. 2021. Relationship Between Sarcopenia and Cardiovascular Diseases in the Elderly: An Overview. Front Cardiovasc Med 8:743710. https://doi.org/10.3389/fcvm.2021.743710
He Y, Li Z, Niu Y, Duan Y, Wang Q, Liu X, Dong Z, Zheng Y, Chen Y, Wang Y, Zhao D, Sun X, Cai G, Feng Z, Zhang W, Chen X. 2024. Progress in the study of aging marker criteria in human populations. Front Public Health 12:1305303. https://doi.org/10.3389/fpubh.2024.1305303
Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. 2016. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ Res 118:535–46. https://doi.org/10.1161/CIRCRESAHA.115.307611
Hoong CWS, Chua MWJ. 2021. SGLT2 Inhibitors as Calorie Restriction Mimetics: Insights on Longevity Pathways and Age-Related Diseases. Endocrinology 162:bqab079. https://doi.org/10.1210/endocr/bqab079
Jasienska G. 2009. Reproduction and lifespan: Trade-offs, overall energy budgets, intergenerational costs, and costs neglected by research. Am J Hum Biol 21:524–32. https://doi.org/10.1002/ajhb.20931
Jasienska G, Bribiescas RG, Furberg AS, Helle S, Núñez-de la Mora A. 2017. Human reproduction and health: an evolutionary perspective. Lancet 390:510–20. https://doi.org/10.1016/S0140-6736(17)30573-1
Keil G, Cummings E, de Magalhães, JP. 2015. Being cool: how body temperature influences ageing and longevity. Biogerontology 16:383–97. https://doi.org/10.1007/s10522-015-9571-2
Kirkwood TB. 1977. Evolution of ageing. Nature 270:301–4. https://doi.org/10.1038/270301a0
Kirkwood TB, Holliday R. 1979. The evolution of aging and longevity. Proc R Soc Lond B Biol Sci 205:531–46. https://doi.org/10.1098/rspb.1979.0083
Kirkwood TB, Rose MR. 1991. Evolution of senescence: late survival sacrificed for reproduction. Philos Trans R Soc Lond B Biol Sci 332:15–24. https://doi.org/10.1098/rstb.1991.0028
Knutson KL. 2012. Does inadequate sleep play a role in vulnerability to obesity? Am J Hum Biol 24:361–71. https://doi.org/10.1002/ajhb.22219
Kowald A, Palmer D, Secci R, Fuellen G. 2024. Healthy aging in times of extreme temperatures: Biomedical approaches. Aging Dis15:601–11. https://doi.org/10.14336/AD.2023.0619
Lee HJ, Alirzayeva H, Koyuncu S, Rueber A, Noormohammadi A, Vilchez D. 2023. Cold temperature extends longevity and prevents disease-related protein aggregation through PA28γ-induced proteasomes. Nat Aging 3:546–66. https://doi.org/10.1038/s43587-023-00383-4
Lehmann G, Muradian KK, Fraifeld VE. 2013. Telomere length and body temperature-independent determinants of mammalian longevity? Front Genet 4:111. https://doi.org/10.3389/fgene.2013.00111
Lemaître JF, Moorad J, Gaillard JM, Maklakov AA, Nussey DH. 2024. A unified framework for evolutionary genetic and physiological theories of aging. PLoS Biol 22:e3002513. https://doi.org/10.1371/journal.pbio.3002513
Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, Hou L, Baccarelli AA, Stewart JD, Li Y, Whitsel EA, Wilson JG, Reiner AP, Aviv A, Lohman K, Liu Y, Ferrucci L, Horvath S. 2018. An epigenetic biomarker of aging for lifespan and healthspan. Aging 10:573–91. https://doi.org/10.18632/aging.101414
Li FX, Xu F, Li CC, Lei LM, Shan SK, Zheng MH, Lin X, Guo B, Tang KX, Duan JY, Wu YY, Cao YC, Liu JJ, Yuan LQ. 2024. Cold Exposure Alleviates T2DM Through Plasma-Derived Extracellular Vesicles. Int J Nanomedicine 19:10077–95. https://doi.org/10.2147/IJN.S441847
Li Y, Tian X, Luo J, Bao T, Wang S, Wu X. 2024. Molecular mechanisms of aging and anti-aging strategies. Cell Commun Signal 22:285. https://doi.org/10.1186/s12964-024-01663-1
Longo VD, Mitteldorf J, Skulachev VP. 2005. Programmed and altruistic ageing. Nat Rev Genet 6:866–72. https://doi.org/10.1038/nrg1706
Longo VD, Anderson RM. 2022. Nutrition, longevity and disease: From molecular mechanisms to interventions. Cell 185:1455–70. https://doi.org/10.1016/j.cell.2022.04.002
Lu SH, Leasure AR, Dai YT. 2010. A systematic review of body temperature variations in older people. J Clin Nurs 19:4–16. https://doi.org/10.1111/j.1365-2702.2009.02945.x
Mahoney SA, Venkatasubramanian R, Darrah MA, Ludwig KR, VanDongen NS, Greenberg NT, Longtine AG, Hutton DA, Brunt VE, Campisi J, Melov S, Seals DR, Rossman MJ, Clayton ZS. 2024. Intermittent supplementation with fisetin improves arterial function in old mice by decreasing cellular senescence. AgingCell23:e14060. https://doi.org/10.1111/acel.14060
Maklakov AA, Chapman T. 2019. Evolution of ageing as a tangle of trade-offs: energy versus function. Proc Biol Sci 286:20191604. https://doi.org/10.1098/rspb.2019.1604
Martin-Ruiz C, Jagger C, Kingston A, Collerton J, Catt M, Davies K, Dunn M, Hilkens C, Keavney B, Pearce SH, den Elzen WP, Talbot D, Wiley L, Bond J, Mathers JC, Eccles MP, Robinson L, James O, Kirkwood TB, von Zglinicki T. 2011. Assessment of a large panel of candidate biomarkers of ageing in the Newcastle 85+ study. Mech Ageing Dev 132:496–502. https://doi.org/10.1016/j.mad.2011.08.001
McGann KP, Marion GS, Camp L, Spangler JG. 1993. The influence of gender and race on mean body temperature in a population of healthy older adults. Arch Fam Med 2:1265–7. https://doi.org/10.1001/archfami.2.12.1265
Mitchell SE, Simpson M, Coulet L, Gouedard S, Hambly C, Morimoto J, Allison DB, Speakman JR. 2024. Reproduction has immediate effects on female mortality, but no discernible lasting physiological impacts: A test of the disposable soma theory. Proc Natl Acad Sci 121:e2408682121. https://doi.org/10.1073/pnas.2408682121
Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. 2010. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab 24:775–84. https://doi.org/10.1016/j.beem.2010.08.014
Nilsson G, Hedberg P, Öhrvik J. 2014. White blood cell count in elderly is clinically useful in predicting long-term survival. J Aging Res 2014:475093. https://doi.org/10.1155/2014/475093
Obermeyer Z, Samra JK, Mullainathan S. 2017. Individual differences in normal body temperature: longitudinal big data analysis of patient records. BMJ 359:j5468. https://doi.org/10.1136/bmj.j5468
Öngel ME, Yıldız C, Akpınaroğlu C, Yilmaz B, Özilgen M. 2021. Why women may live longer than men do? A telomere-length regulated and diet-based entropic assessment. Clin Nutr 40;1186–91. https://doi.org/10.1016/j.clnu.2020.07.030
Page EB. 1963. Ordered Hypotheses for Multiple Treatments: A Significance Test for Linear Ranks. Journal of the American Statistical Association 58:216–30. https://doi.org/10.2307/2282965
Palani SN, Sellegounder D, Wibisono P, Liu Y. 2023. The longevity response to warm temperature is neurally controlled via the regulation of collagen genes. Aging Cell 22:e13815. https://doi.org/10.1111/acel.13815
Picca A, Pesce V, Lezza AMS. 2017. Does eating less make you live longer and better? An update on calorie restriction. Clin Interv Aging 12:1887–902. https://doi.org/10.2147/CIA.S126458
Proctor MJ, McMillan DC, Horgan PG, Fletcher CD, Talwar D, Morrison DS. 2015. Systemic inflammation predicts all-cause mortality: a Glasgow inflammation outcome study. PLoS One 10:e0116206. https://doi.org/10.1371/journal.pone.0116206
Rikke BA, Johnson TE. 2004. Lower body temperature as a potential mechanism of life extension in homeotherms. Exp Gerontol 39:927–30. https://doi.org/10.1016/j.exger.2004.03.020
Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, Muller D, Metter EJ. 2002. Biomarkers of caloric restriction may predict longevity in humans. Science 297:811. https://doi.org/10.1126/science.1071851
Ruggiero C, Metter EJ, Cherubini A, Maggio M, Sen R, Najjar SS, Windham GB, Ble A, Senin U, Ferrucci L. 2007. White blood cell count and mortality in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol 49:1841–50. https://doi.org/10.1016/j.jacc.2007.01.076
Ruggiero C, Metter EJ, Melenovsky V, Cherubini A, Najjar SS, Ble A, Senin U, Longo DL, Ferrucci L. 2008. High basal metabolic rate is a risk factor for mortality: the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci 63:698–706. https://doi.org/10.1093/gerona/63.7.698
Sayer AA, Kirkwood TB. 2015. Grip strength and mortality: a biomarker of ageing? Lancet 386:226–7. https://doi.org/10.1016/S0140-6736(14)62349-7
Shapiro SS, Wilk MB. 1965. An analysis of variance test for normality (complete samples). Biometrika 52:591–611. https://doi.org/10.2307/2333709
Simonsick EM, Meier HCS, Shaffer NC, Studenski SA, Ferrucci L. 2016. Basal body temperature as a biomarker of healthy aging. AGE 38:445–54. https://doi.org/10.1007/s11357-016-9952-8
Smith L, Yang L, Hamer M. 2019. Handgrip strength, inflammatory markers, and mortality. Scand J Med Sci Sports 29:1190–6. https://doi.org/10.1111/sms.13433
Soare A, Cangemi R, Omodei D, Holloszy JO, Fontana L. 2011. Long-term calorie restriction, but not endurance exercise, lowers core body temperature in humans. Aging 3:374–9. https://doi.org/10.18632/aging.100280
Speakman JR. 2020. Why does caloric restriction increase life and healthspan? The ‘clean cupboards’ hypothesis. Natl Sci Rev 7:1153–6. https://doi.org/10.1093/nsr/nwaa078
Sultanova Z, Ivimey-Cook ER, Chapman T, Maklakov AA. 2021. Fitness benefits of dietary restriction. Proc Biol Sci 288:20211787. https://doi.org/10.1098/rspb.2021.1787
Waalen J, Buxbaum JN. 2011. Is older colder or colder older? The association of age with body temperature in 18,630 individuals. J Gerontol A Biol Sci Med Sci 66:487–92. https://doi.org/10.1093/gerona/glr001
Waziry R, Ryan CP, Corcoran DL, Huffman KM, Kobor MS, Kothari M, Graf GH, Kraus VB, Kraus WE, Lin DTS, Pieper CF, Ramaker ME, Bhapkar M, Das SK, Ferrucci L, Hastings WJ, Kebbe M, Parker DC, Racette SB, Shalev I, Schilling B, Belsky DW. 2023. Effect of long-term caloric restriction on DNA methylation measures of biological aging in healthy adults from the CALERIE trial. Nat Aging 3:248–57. https://doi.org/10.1038/s43587-022-00357-y
Ziomkiewicz A, Sancilio A, Galbarczyk A, Klimek M, Jasienska G, Bribiescas RG. 2016. Evidence for the Cost of Reproduction in Humans: High Lifetime Reproductive Effort Is Associated with Greater Oxidative Stress in Post-Menopausal Women. PLoS One 11:e0145753. https://doi.org/10.1371/journal.pone.0145753
Final information
Acknowledgements
The authors are grateful to the two anonymous reviewers for their meticulous and thoughtful comments, which significantly strengthened the scientific rigor and clarity of this article.
Contributions from individual authors
PPC developed and designed the study, collected the data, performed the statistical analyses, conducted the literature search and collected all pertinent references, interpreted the results, drafted the initial version of the manuscript and all subsequent versions, as well as produced all figures and tables for this manuscript. KC planned and managed the research project, collected the data, and contributed to the critical review of the manuscript. All authors have read and approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Financial disclosure
This research did not receive any funding and was self-sponsored by the authors.
Conflict of interest
The authors have no conflicts of interest to declare.
We certify that this manuscript represents entirely original work that has not been published previously or concurrently submitted elsewhere.
Corresponding author
Piotr Paweł Chmielewski, Division of Anatomy, Department of Human Morphology and Embryology, Faculty of Medicine, Wroclaw Medical University, Wroclaw, Poland, 6a Chalubinskiego Street, 50-368 Wroclaw, Poland, e-mail: piotr.chmielewski@umw.edu.pl