Introduction
Health disparities refer to the differences in health between groups resulting from socioeconomic and environmental disadvantages; these disparities occur across many categories, including ethnic groups, gender, socioeconomic roles, and disability status, which result in apparent health inequalities across such divisions (Braveman 2006). The disparity in health outcomes/care between males and females has been of special interest because it is a natural biological problem in health policy making (Wamala and Lynch 2002; Ostrowska 2012). In addition to its significance in health policy, sex/gender-based inequality also interests bioarchaeologists. These researchers are interested in examining this disparity in relation to male and female lifestyles and socioeconomic roles played during ancient times, and how this sex-gender-based difference has influenced health and social equality in human society (Schepartz et al. 2017). Initial differences between males and females have been explained using the biomedical model, i.e., that postmenopausal women have higher risks because of their higher prevalence of osteoporosis (Cummings et al. 1985, 1989; Cooper et al. 1992; Cummings and Melton 2002). However, this trend has been complicated by social socioeconomic stratification during recent human evolution. For example, it has been shown that the hierarchical nature of an ancient Greek population informed gender-based inequalities which affected dietary differences and dental health (Schepartz et al. 2017). Thus, epidemiological studies on sex-based health inequalities should combine biomedical and socioeconomic models to determine factors for perpetuating these differences (and how they are linked). In this research, we examined the age-related changes of the spinal column. Our work elucidates that sex/gender-based differences in physiological and physical stresses influence the etiology and pathophysiology of spinal degenerative diseases in a bioarcheological setting. Age-related changes of the vertebral column are generally advanced by daily work activities (i.e. heavy labor) or by chronic and habitual stress (Merbs 1996; Katzman et al. 2010; Pili et al. 2018; Aguirre et al. 2020; Yustos et al. 2021). Weposit that it is beneficial to study male-female differences in the age-related change patterns of the vertebral column in light of the male-female differences in biological and sociocultural elements.
As a result of the natural ageing process, vertebrae of the spine experience several changes, including a decrease in bone mass which may lead to osteopenia and osteoporosis, as well as an increase in degenerative joint diseases (DJD). The latter category involves problems such as osteoarthritis, degenerative disk disease, calcification of associated ligaments, and weakening of attached muscles (Benoist 2003). These changes eventually lead to a number of “painful and debilitating disorders” (Papadakis et al. 2011), including discomfort, pain in individual sections or the whole of the spine, or loss in mobility. Osteophytosis and Vertebral Compressive Fracture (VCF) are two primary outcomes of these age-related changes and pathological processes. While VCF is one of the most common symptoms of osteoporosis (Cummings et al. 1989), osteophytic formation is an adaptive bone remodeling response to daily life stressors and the progressive reduction of bone mineral density and mass, which increases load-bearing surface areas (Fraser et al. 1997). Other less studied consequences of age-related changes in the spine are arthritis of the vertebral body and facet joint osteoarthritis, caused by a combination of multiple factors (i.e. amount of use, profession-related overload, age-related changes, genetics, and injury). Symptoms of spinal osteoarthritis include pain, swelling, and loss of flexibility (Dreyfuss 1994; Manchikanti et al. 2004).
The age-related changes of vertebral bodies have been widely studied in contemporary, historical, and prehistoric populations. Different health outcomes have been linked to nutrition, lifestyles involving poor posture and incorrect ergonomics, levels of sex hormones, trauma, and labor activities (Stewart 1958; Chapman 1972; Burrell et al. 1986; Riggs et al. 1986; Miller et al. 1988; Jurmain 1990; Waldron 1991; Lovell 1994; Knusel et al. 1997; Sofaer-Derevenski 2000; Brickley 2002; Steckel and Rise 2002; Fillingim 2003; Manchikanti et al. 2004; Leveille et al. 2005; Van der Merwe et al. 2006; Rojas-Sepulveda et al. 2008; Bailey 2009; Novak and Slaus 2011; Kim et al. 2012; Shimoda et al. 2012; Zukowski et al. 2012; Missikpode et al. 2015; Hou et al. 2017; Steckel et al. 2019). Specifically, attention has been called for “activity-related osseous change” in historical populations. For example, a comparison study of populations from Wharram Percy (a medieval site) and Ensay (a 16th –19th century site) in the U.K. reveals that sex-based differences in osseous changes of the spine; these findings have been related to dissimilarities in physioanatomy and life history (i.e., females undergoing child bearing and menopause) along with gender-based divisions of labor (i.e., males undertaking heavy-duty labor) (Sofaer-Derevenski 2000). Thus, we propose that differing age-related and spinal osseous changes between sexes could be important indicators in differentiating between gender-based socioeconomic roles in ancient human societies. Knowledge of how physiological and physical sex-based differences affect the etiology and pathophysiology of skeletal degenerative diseases can assist in distinguishing these elements from other sociocultural factors that may have contributed to health disparities in bioarchaeological studies.
In this study, sex-based differences in the ageing of the spine were tested in human skeletons excavated from a Bronze Age cemetery of the Western Zhou Dynasty (1045–771 BCE), at the Dahekou site (Baidu Map: N35.749438°, E111.788635°) located in Yicheng County, Shanxi Province, China (Fig. 1). Between 2007 and 2017, approximately 2,200 tombs were excavated during thirteen phases. Funeral objects were found, including bronze vessels, pottery, jade bone tools, lacquered wood items, tin vessels, shell tools, and sacrificed dog remains (Xie et al. 2011; Guo 2015; Li et al. 2021). From the inscriptions on bronze funeral vessels, scholars realized the existence of a small city-state Ba during the Western Zhou Dynasty (1045–771 BCE), and that this cemetery was used by people of the State Ba (Xie et al. 2011). The State Ba was a small city-state, but had no record in the history scrolls for the Western Zhou Dynasty. The density and numbers of tombs found at the Dahekou cemetery indicates that it was in an urban setting with a relatively large population size, most likely the capital city of the recovered Ba State.
Fig. 1. Location of the Dahekou site.
The socioeconomic structure during the Western Zhou dynasty was a form of feudalism similar to the socio-economic mode of medieval Western Europe (Feng 2014), in which the king owned all of the land and lived in the capital city. Lords of regional states lived in towns and controlled the land, while the peasants lived outside of the towns and worked on the land. Future archaeological study of the Dahekou site would help in reconstructing the daily life of its ancient inhabitants. In total, the remains of 2201 individuals (549 in excavation areas one to six, and 1652 from excavation areas seven to thirteen) were excavated from the Dahekou site (Han 2019). The sex of 1762 individuals was identified among this group. There were 873 males and 889 females, suggesting a balanced male vs. female ratio (0.982), or no significant loss of male individuals. The ratio was a sign of a rather peaceful period for this ancient Chinese region. Our preliminary study also revealed that there were very low cranial fractures (i.e., only two males showed signs of skull fractures: one in the mandible, the other one along a nasal bone). This finding indicated a low incidence of intentional interpersonal violence (Lovell 1994; Zhang et al. 2021), and peaceful existence in Ba State society. (Appendix Table 1). Thus, the Dahekou site provided a revealing window into an ancient urban population with minimal disturbance from interpersonal conflicts for our ageing analysis. In this study, we investigated the influence of sex-based differences and health inequalities on the age-related changes of the vertebral column, including fractures and osteoarthritis. Accordingly, we showed how sex-based physioanatomy and a gender-based division of labor affected the spinal column during a relatively peaceful time of feudalism.
Materials and methods
The skeletons from excavation phases seven to thirteen were used in this study (Table 1). Based on current archeological evidence, the section (excavation areas seven to thirteen) at the Dahekou cemetery was an area used by the commoners (1652 skeletons in total), not the social elites of Ba State; they are proposed to have engaged in an urban lifestyle within the walls of a city, likely the capital of the Ba State. The spines were well preserved in 120 of the skeletons, with nearly 90% of vertebrae from the three segments combined (i.e., cervical, thoracic, and lumbar) (Appendix Table 2). Sex was determined using skeletal features from the skull (including the mastoid process, supraorbital ridge and glabella, the nuchal crest, and the mental eminence). The pelvis was also used (including the morphology of the medial margin of the inferior pubic branch, the subpubic angle, and the greater sciatic notch) following Buikstra and Ubelaker (1994). Age at death was estimated by grading the auricular surface degeneration of the pelvis (Lovejoy et al. 1985). Furthermore, age at death was categorized into three periods based on eight phases: one to two as a young adult (twenty to thirty-five years old), three to six as a middle adult (thirty-six to fifty years old), and seven to eight as an older adult (>50 years). Age was then examined using dental wear stages (Lovejoy 1985), with consideration of the phenomenon that wear is more advanced in pre-contemporary populations due to a more abrasive diet (Molnar 1972; Kaidonis 2008). At Dahekou, there were one hundred and twenty full adult skeletons (forty-six males and seventy-four females) with good preservation from phases seven to thirteen (areas excavated at different times).
Individuals with an age at death estimated at ≥20 years with a reliable determination of sex were selected from two age groups: young adults (Adult Young or AY: 20–35 years) and middle-aged adults (Adult Middle or AM: 36–50 years). The objective was to examine the frequency of degenerative diseases such as VCF and osteophytosis at both the vertebral body and joint facets. Individuals in the Old Adult (>50 years) category were not possible to study due to unfavorable postmortem vertebral preservation conditions.
| Adult Young (20–35 Years) | Adult Middle (36–50 Years) | Sum | ||||
| VCF+VBO | JFO | VCF+VBO | JFO | VCF+VBO | JFO | |
| Male | 19 | 9 | 27 | 13 | 46 | 22 |
| Female | 42 | 41 | 32 | 30 | 74 | 71 |
| TOTAL | 61 | 50 | 59 | 43 | 120 | 93 |
A macroscopic visual analysis of each vertebra was carried out, and VCF was recorded with the identification of vertebrae (i.e., T10 or L5) and their type of fracture (i.e., wedge, biconcavity, or crush deformity fracture).
Next, osteophytes on the edge of the vertebral body were investigated and graded in the cervical, thoracic, and lumbar vertebrae. The samples were graded using a five-stage standard following Snodgrass (2004) and Van der Merwe et al. (2006) (Fig. 2). Briefly, Grade 0 has no signs of osteophytosis, and Grade 1 has osteophyte points or slight lipping on the vertebral body margins. Grade 2 has more lipping on the margins (no more than 2mm projecting horizontally from the vertebral body), while Grade 3 exhibits advanced lipping projecting horizontally from the vertebral body at larger than 2mm or almost fusion of the osteophytes. Grade 4 exhibits the fusion of osteophytes on adjacent vertebrae. In order to compare the severity of osteophytosis in different vertebral divisions, the mean osteophytic value (MOV) was calculated as the mean osteophytic grade value for each vertebra (grades of the upper and lower margins) in different sex-age groups. In total, vertebrae (C1 to L5) from 120 individuals in four sex-age groups were scored (Table 1).
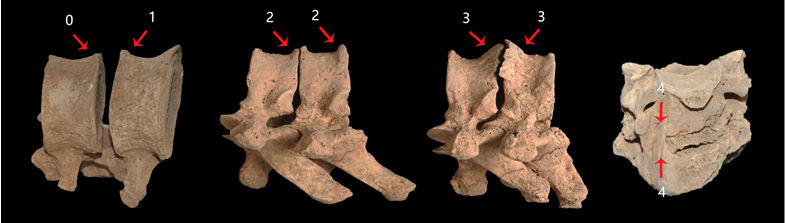
Fig. 2. Five stages of osteophytosis (vertebral body). All specimens were from the Dahekou site. From left to right: M9363 L1-L2 (Grades 0 and 1), M9045 T7-T8 (Both Grade 2), M9045 T9-T10 (Both Grade 3), M7215 C5-C6 (Both Grade 4). Images not scaled.
Lastly, signs of degenerative joint diseases were investigated and graded in the facet joints of cervical and thoracic vertebrae (details given below). The facet joints in the spine (zygapophysial joints) are synovial joints between the articular processes of two adjacent vertebrae. Facet joint osteoarthritis has been investigated intensively inmedicine due to its association with neck and back pain (Manchikanti et al. 2004). Facet joints render the spine flexible; in each spinal motion segment, there are two facet joints formed by neighboring vertebrae. Thus, a vertebra has two superior (left and right) and two inferior (left and right) joint facets. Facet joint osteoarthritis is attained when these joints become inflamed and painful due to the degeneration of intervertebral discs and development of vertebral body osteoarthritis (Manchikanti et al. 2004). Age-related changes in the intervertebral disc lead to narrowing of the intervertebral space (Adams and Dolan 2012), which results in pressure overload of the facet joints and destruction of joint cartilage. This situation gives rise to localized facet joint osteoarthritis (Manchikanti et al. 2004), in addition to vertebral body osteoarthritis. In this study, we established a five-stage standard (Fig. 3) scoring system for this condition which has been explained earlier.
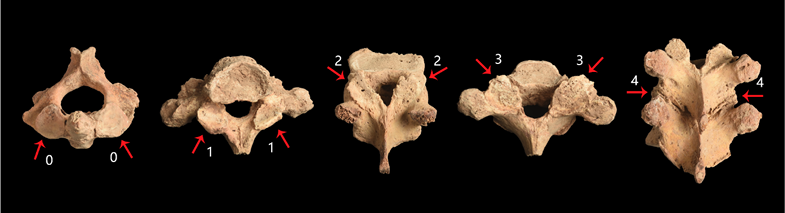
Fig. 3. Five stages of facet joint osteoarthritis. All specimens were from the Dahekou site. From left to right: M12288 C2 superior joint facets (Grades 0), M12026 T1 inferior joint facets (Grade 1), M12125 T11 superior joint facets (Grade 2), M12026 T1 superior joint facets (Grade 3), M12125 T9-T10 fused (Grade 4). Images not scaled.
Our facet osteoarthritis investigation was limited to the cervical and thoracic segments (C1 to T12), and the lumbar segment was not included due to unfavorable postmortem preservation of the joint facets in lumbar vertebrae. The number of individuals suited for this section of the investigation was smaller and more imbalanced. There were twenty-two males and seventy-one females (reasons for the less favorable postmortem preservation of joint facets in males compared to females were unknown). Both the superior and inferior facets of the left and right sides were recorded.
Data was then analyzed using a statistical analysis program named GraphPad 6.0 for Windows (GraphPad Software Inc., La Jolla, CA). Mean scores of osteophytosis in a vertebra were based on the means of the upper and lower margins of the same vertebra or four joint facets of the same vertebra. Mean scores of the segments were means of mean scores of all vertebrae preserved in a segment. Group differences in prevalence/frequencies were compared between the different sex-age groups using a non-parametric Chi-square for independence in a contingency table. The significance level was set at α = 0.05.
Results
Vertebral Compressive Fracture (VCF)
In total, forty vertebrae (1.6% in terms of prevalence per vertebra) in twenty-one individuals (17.5% in terms of prevalence per individual) carried signs of VCF (Table 2; Appendix Table 3). Two types of compressive deformity factures were observed in the Dahekou population: wedge deformity fractures (anterior height < middle height < posterior height) and crush deformity fractures (in which the whole body was compressed evenly) (Appendix Table 3; Fig. 4). Half of the VCFs (twenty out of forty) were wedge deformity fractures, and the other half were crush deformity fractures. Nevertheless, there were no biconcavity deformity fractures. Nearly half of the individuals (47.6%) had multiple fractures in separated or continuous vertebrae, mostly in the thoracolumbar spine (Appendix Table 3). The prevalence of continuous fractures was 28.6% (two out of seven individuals with VCF) in the female young adult (AY) group, 36.4% (four out of eleven individuals with VCFs) in female middle adult (AM) group, and 33.3% (one out of three individuals with VCF) in male AM group (either wedge or crush deformity varieties). No VCF was found in male AY (0%).
| Cervical | Thoracic | Lumbar | Sum | |
| Female Adult Young | 0 | 0.2% | 4.5% | 1.1% |
| Female Adult Middle | 1.0% | 1.2% | 12.8% | 3.6% |
| Male Adult Young | 0 | 0 | 0 | 0 |
| Male Adult Middle | 0 | 1.0% | 1.4% | 0.9% |
| Total | 0.3% | 0.5% | 3.7% | 1.6% |
Among the group of forty VCFs found, most were in the lumbar segment (82.2%), followed by thoracic (11.1%) and cervical (6.7%) segments (Table 2; Fig. 4). In particular, the majority of the locations were in the lower lumbar vertebrae. Of the seventeen individuals with lumbar VCF, L5 was involved in fifteen cases and eight of these were isolated to L5 alone (categorized as either the aforementioned wedge or crush deformity). As opposed to the lower lumbar vertebrae, a cervical segment was involved in only one individual (C5-6 from M9054, female, age at death around 40 years) (Appendix Table 3).

Fig. 4. Compressive vertebral fracture. Left: M9066, female, L1 Wedge deformity fracture; Right: M9066, female, L2 Crush deformity fracture. Scale: 10mm per segment.
The VCF prevalence per vertebra was higher in females than in males (Table 2). Throughout this sample, 10 out of 896 vertebrae (1.1%) in the female AY group, 25 out of 687 vertebrae (3.6%) in the female AM group, and 5 out of 588 vertebrae (0.9%) in the male AM group were involved in VCF. Remarkably, no vertebrae from the male AY group showed signs of VCF, indicating the impact of sex-age status (Chi-square test: X2 = 29.0, P <0.0001, DF =3). The lumbar segment had the highest involvement concerning VCF prevalence per vertebral segment. The VCF prevalence was 0.3% in cervical, 0.5% in thoracic, and 3.7% in lumbar segments, demonstrating the effect of position in VCFs (Chi-square test: X2 = 66.9, P<0.0001, DF =2).
Concerning VCF prevalence per individual (Table 2; Appendix Table 2), females had a higher VCF prevalence than males (Female 18 out of 74 or 24.3% vs. Male 3/46 or 6.5%; Chi-square test: X2 = 6.23, P=0.0013, DF =1). In addition, the prevalence of VCF was higher in the female AM group than in the female AY group. In males, no cases were found in the Male AY group and the prevalence was 11.1% in the Middle Adult group (35-45± years); in females, VCF prevalence was at 16.7% in the Adult Young group and 34.4% in the Middle Adult group (Table 2; Appendix Table 2). In the female Adult Young group, six of the seven individuals were clustered in an age range between twenty-five and thirty years old.
Vertebral Osteophytosis
Cervical, thoracic, and lumbar osteophytosis were observed in all sex-age groups (Tables 5-9). Males tended to have more vertebrae affected in their spine than females in two age groups (Table 3). In terms of mean osteophytic grades, there was a trend that osteophytic formation increased from young to middle adults. However, males expressed higher overall osteophytic stages in all vertebral segments than females (Table 3; Fig. 5a-b). For example, the prevalence of Grade 2 and above was 21.9% vs. 10.5% in the young adult group, while it was 34.7% vs. 19.7% in the middle adult group (Table 3).
| Cervical | Thoracic | Lumbar | SUM | |||||||
| Male | Female | Male | Female | Male | Female | Male | Female | Combined | ||
| Osteophytosis grade | Adult Young | 0.63 ± 1.00 | 0.38 ± 0.76 | 0.76 ± 0.80 | 0.42 ± 0.55 | 1.13 ± 1.11 | 1.08 ± 1.03 | 0.80 ± 0.92 | 0.55 ± 0.79 | 0.63 ± 0.84 |
| Adult Middle | 0.87 ± 1.27 | 0.56 ± 0.91 | 1.05 ± 1.13 | 0.51 ± 0.68 | 1.94 ± 1.08 | 1.53 ± 1.13 | 1.20 ± 1.23 | 0.74 ± 0.95 | 0.95 ± 1.11 | |
| Prevalence of Grade 2 and above | Adult Young | 26 (22.2%) | 25 (10.2%) | 35 (16.8%) | 14 (3.1%) | 28 (34.1%) | 55 (27.8%) | 89 (21.9%) | 94 (10.5%) | 183 (14.0%) |
| Adult Middle | 40 (24.2%) | 31 (16.2%) | 77 (26.6%) | 33 (9.51%) | 87 (64.9%) | 71 (48.0%) | 204 (34.7%) | 135 (19.7%) | 339 (26.6%) | |
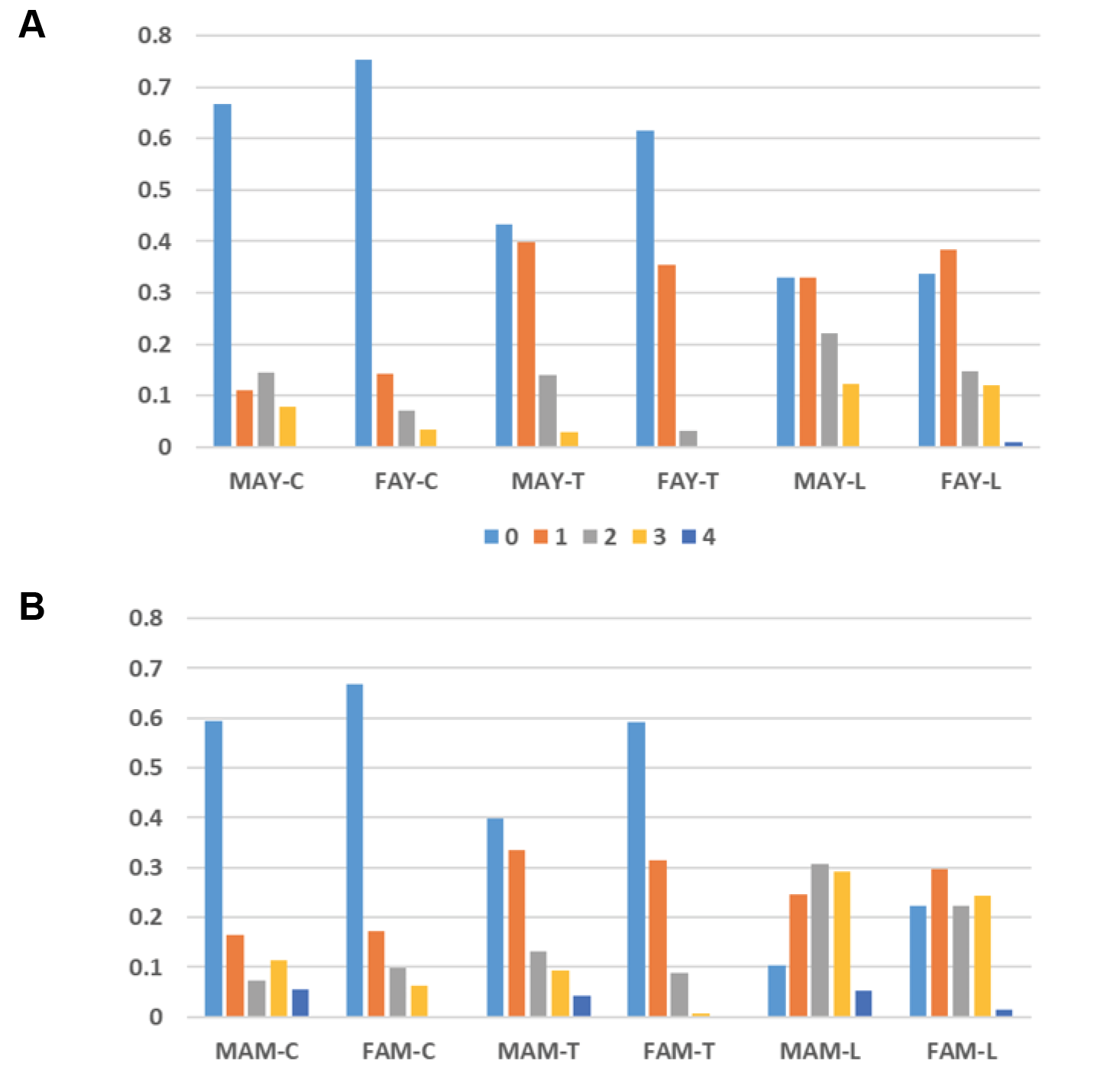
Fig. 5. Osteophyte grades for cervical, thoracic and lumbar segments in male and female adult young groups (a) and male and female adult middle groups (b). There was a trend that the osteophytic formation increased from young to middle adults, and that the males expressed higher overall osteophytic stages in three vertebral segments than females. Abbreviations: MAY – Male Adult Young; FAY – Female Adult Young; MAM – Male Adult Middle; FAM – Female Adult Middle.
In addition, males and females had similarly positioned patterns of vertebral osteophytosis in terms of Grade 2 and above expressions. For instance, we detected thoracic < cervical < lumbar, except in male AM cervical < thoracic, while the percentage of Grade 2 and above was higher in males than in females (Tables 4–5). In different sex-age groups, 21.9% (89 out of 407) in the male AY group exhibited Grade 2 and above, whereas 10.5% (94 out of 896) in the female AY group exhibited Grade 2 and above. Furthermore, 34.7% (204 out of 588) of vertebrae in the male AM group exhibited Grade 2 and above, while 19.6% (135 out of 1275) in the female AM group exhibited Grade 2 and above (combined: AY – 14.0% or 183 out of 1303; AM - 26.6% or 339 out of 1275).
| Osteophyte Grade (Adult Young) | Cervical | Thoracic | Lumbar | |||
| Male | Female | Male | Female | Male | Female | |
| 0 | 78 (66.7%) | 184 (75.4%) | 90 (43.3%) | 279 (61.5%) | 27 (32.9%) | 67 (33.8%) |
| 1 | 13 (11.1%) | 35 (14.3%) | 83 (39.9%) | 161 (35.5%) | 27 (32.9%) | 76 (38.4%) |
| 2 | 17 (14.5%) | 17 (7.0%) | 29 (13.9%) | 14 (3.1%) | 18 (22.0%) | 29 (14.6%) |
| 3 | 9 (7.7%) | 8 (3.3%) | 6 (2.9%) | 0 (0.0%) | 10 (12.2%) | 24 (12.1%) |
| 4 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (1.0%) |
| N | 117 | 244 | 208 | 454 | 82 | 198 |
| Mean | 0.63 | 0.38 | 0.76 | 0.42 | 1.13 | 1.08 |
| SD | 1.00 | 0.76 | 0.80 | 0.55 | 1.11 | 1.03 |
| Osteophyte Grade (Adult Middle) | Cervical | Thoracic | Lumbar | |||
| Male | Female | Male | Female | Male | Female | |
| 0 | 98 (59.4%) | 128 (66.7%) | 115 (39.8%) | 205 (59.1%) | 14 (10.4%) | 33 (22.3%) |
| 1 | 27 (16.6%) | 33 (17.2%) | 97 (33.6%) | 109 (31.4%) | 33 (24.6%) | 44 (29.7%) |
| 2 | 12 (7.3%) | 19 (9.9%) | 38 (13.1%) | 31 (8.9%) | 41 (30.6%) | 33 (22.3%) |
| 3 | 19 (11.5%) | 12 (6.3%) | 27 (9.3%) | 2 (0.6%) | 39 (29.1%) | 36 (24.3%) |
| 4 | 9 (5.5%) | 0 (0.0%) | 12 (4.2%) | 0 (0.0%) | 7 (5.2%) | 2 (1.4%) |
| N | 165 | 192 | 289 | 347 | 134 | 148 |
| Mean | 0.87 | 0.56 | 1.05 | 0.51 | 1.94 | 1.53 |
| SD | 1.27 | 0.91 | 1.13 | 0.68 | 1.08 | 1.13 |
Overall, the lumbar spine had the highest prevalence of osteophytes that were at Grade 2 and above in the Adult Middle groups, yielding 64.9% in males and 48.0% in females (Appendix Table 4). Specifically, the male Adult Young group had a high prevalence of osteophytosis (Grade 2 or above) in C5-7 (33.3–44.4%), T6-12 (16.7–31.6%), and L1-5 (17.6–43.8%), with L3 and L5 displaying the highest prevalence (43.8%). The male Adult Middle group had high values from C3-7 (18.2–41.7%), with 41.7% in C6 to higher values from T10 (44.0%) and L4 (77.8%). In the female Adult Young group, high values were from C5-6 (22.0–25.5%) and L2-5 (22.5 to 42.5%), while they were generally low in the thoracic segment. However, the female Adult Middle group demonstrated high values from C5-6 (40.7%), while they were 33.3–62.1% from L1-5 and 20.0% from T8-9 (Appendix Table 4).
In terms of the mean osteophytic value (MOV), different sex-age groups had different patterns, yet all lumbar elements, lower thoracic elements, C5, and C6 generally exhibited high stages of osteopathic formation (Appendix Table 5; Fig. 6). In the male Adult Young group, the five top values were as followed (in the same decreasing order hereafter): L5, L4, L3, T9, and C5 (range 1.38–1.06). Moreover, the female Adult Young group had top five figures from L4, L5, L3, L2, and C6 (range 1.45–1.06). Additionally, the top five calculations in the male Adult Middle category were from L4, L3, L2, L5, and L1 (range 2.19–1.59), followed by T10, C6, T11, T9, and C5 (range 1.52–1.26). By comparison, the top five values in the female Adult Middle group were in L5, L4, L3, L2, and C5 (range 1.83–1.26), followed by L1, C6, T7, T8, and T10 (1.20-0.73).
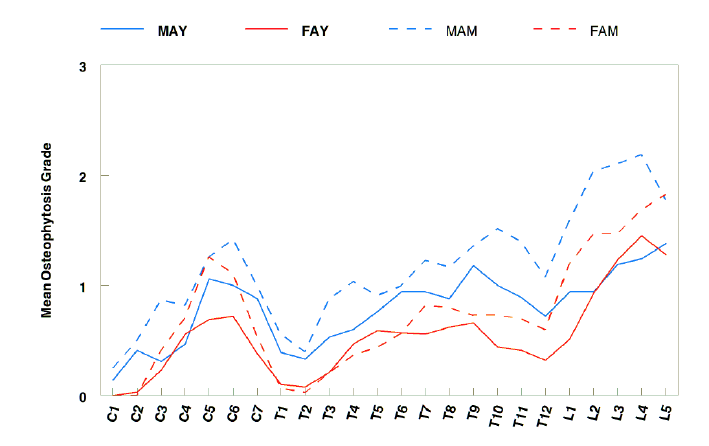
Fig. 6. The mean osteophytic value (MOV) (mean osteophyte grades of individual vertebra) in the four sex-age groups for the Dahekou skeletons. Abbreviations: MAY – Male Adult Young; FAY – Female Adult Young; MAM – Male Adult Middle; FAM – Female Adult Middle.
Facet Joint Osteoarthritis
Facet joint osteoarthritis was investigated in 6082 joint facets from 1520 vertebrae in twenty-two adult males (nine in the young group and thirteen in the middle group), as well as in seventy-one adult females (forty-one in the young group and thirty in the middle group). Overall, the prevalence of joint facet arthritis was relatively low: 235 of 6082 joint facets (3.9%) exhibited osteoarthritis at Grade 2 or above (Grade 2: 140 or 2.3%, Grade 3: 77 or 1.3%, and Grade 4: 18 or 0.3%). Generally, the percentages of Grade 2 and above were higher in males than in age-matched females (Table 6). Accordingly, the prevalence and severity of facet joint osteoarthritis was low in the female groups. However, signs of joint facet osteoarthritis were more prevalent in the male groups. In fact, the male Adult Young group had relatively higher thoracic joint facet arthritis, while the male Adult Middle group had higher cervical joint facet arthritis (Appendix Table 6). The side difference in affected bone was unremarkable in females. By comparison, males expressed a general trend in which the left side had higher severity of facet joint osteoarthritis than the right side in both young and middle adult groups; this pattern was especially high in C3 and C7 (left > right) within the male Middle Adult groups (Fig. 7; Appendix Table 6).
| Male Adult Young (Individual N=9) | Female Adult Young (Individual N=41) | Male Adult Middle (Individual N=13) | Female Adult Middle (Individual N=30) | |||||
| Left | Right | Left | Right | Left | Right | Left | Right | |
| 0 | 230 (79.9%) | 225 (78.1%) | 1220 (91.7%) | 1220 (91.7%) | 382 (82.5%) | 374 (80.8%) | 826 (86.0%) | 824 (85.8%) |
| 1 | 30 (10.4%) | 39 (13.5%) | 101 (7.6%) | 101 (7.6%) | 31 (6.7%) | 48 (10.4%) | 92 (9.6%) | 104 (10.8%) |
| 2 | 17 (5.9%) | 14 (4.9%) | 5 (0.4%) | 4 (0.3%) | 27 (5.8%) | 24 (5.2%) | 25 (2.6%) | 24 (2.51%) |
| 3 | 7 (2.4%) | 8 (2.8%) | 4 (0.3%) | 5 (0.4%) | 17 (3.7%) | 11 (2.4%) | 17 (1.8%) | 8 (0.8%) |
| 4 | 4 (1.4%) | 2 (0.7%) | 0 (0.0%) | 0 (0.0%) | 6 (1.3%) | 6 (1.3%) | 0 (0.0%) | 0 (0.0%) |
| N | 288 | 288 | 1330 | 1330 | 463 | 463 | 960 | 960 |
| Mean | 0.35 | 0.34 | 0.09 | 0.09 | 0.35 | 0.33 | 0.20 | 0.18 |
| SD | 0.81 | 0.76 | 0.33 | 0.34 | 0.85 | 0.79 | 0.57 | 0.5 |

Fig. 7. Side difference of joint facet arthritis in Dahekou skeletons. Abbreviations: MAY – Male Adult Young; FAY – Female Adult Young; MAM – Male Adult Middle; FAM – Female Adult Middle; L – Left; R – Right.
Discussion
There was discordance in the paleoepidemiology of vertebral compressive fractures (VCF) and age-related degenerative joint diseases (DJD) at the Bronze Age Dahekou site. While females had an overall higher VCF prevalence and earlier manifestation of VCF than males, males had a higher severity of DJD than females. These sex-based differences in the patterns of VCF and DJD may indicate different mechanisms for attaining spinal changes in males and females, both naturally and socioeconomically.
Vertebral Fractures in Urban and Agricultural Populations
In contemporary humans, the prevalence of vertebral fractures increases with age for both males and females; postmenopausal women have higher risks because of their higher prevalence of osteoporosis (Cummings et al. 1985, 1989; Cooper et al. 1992; Cummings and Melton 2002). At Dahekou, females had an overall higher VCF prevalence that was not at variance with the modern population, thus suggesting the effects of osteoporosis. Females at Dahekou also demonstrated an earlier manifestation of VCF than males, which may indicate an early onset of age-related changes and osteoporosis within females rather than males. In our opinion, a higher prevalence in females is most likely related to sex-based differences in pathophysiology, a circumstance in which females could have higher hormone deficiency-related osteoporosis than males. Moreover, osteoporotic fractures are uncommon in physically active populations (Jónsson et al. 1992; Sanders et al. 2002; Pisani et al. 2016). In this regard, the low VCF in males at Dahekou may suggest higher physical stress in males among various biological and physical factors. Vice versa, these trends suggest that women at Dahekou were generally involved in low physical stress during their daily lives, a phenomenon of gender-biased divisions of labor that was corroborated by the severity of DJDs (high in males and low in females).
Physioanatomy and life history, along with gendered divisions in socioeconomic statuses and roles (hence different labor-related stressors) might be responsible for the different patterns found at Dahekou. Females from this location generally had high grades of osteophytosis in the lumbar and cervical segments and very low grades in thoracic segments. On the contrary, although males had high lumbar osteophytotic development to a lesser degree, they did exhibit relatively higher osteophytosis at the thoracic area compared to females. This pattern of sex-based differences at the Dahekou site might have been the consequence of a gender-based division of labor: males and females both engaged in physically demanding jobs, yet the types of jobs were different. Hence, what type of labor division did the Dahekou population engage in as a result of its socioeconomic mode?
The age of the Ba State was in the era of the feudalism system. During the economic mode of the feudalism system, civilian men practiced agriculture in rural areas or specialized in craft and service industries within urban areas, including cities and towns (Barford 2005; Agnew et al. 2015). Under this structure, the overall injury patterns could be interpreted in light of feudal socioeconomic relations and class conflict. As mentioned earlier, limited evidence (including the balanced male vs. female ratio and lack of signs for skull injury (Appendix Table 1) indicated a lack of intense interpersonal conflicts in a relatively peaceful period.
Risks of skeletal injuries include occupational risks (i.e., from farm labor in agriculture), environmental health risks (i.e., rugged terrain), and interpersonal conflicts (i.e., war, battles, structural violence, or fights and assaults) (Agnew et al. 2015). There are remarkable differences in VCF prevalence among different populations throughout the Eurasian continent (Table 7), due to differences in settlement patterns, subsistence methods, social stability, age-related factors of change, mortality patterns, and genetic factors. Differences in the frequency of bone fractures among different populations are related to differences in social status (Geber 2015), environment/terrain (Kilgore et al. 1997), occupation (Grauer and Roberts 1996), or economic setting, i.e., rural vs. urban (Agnew et al. 2015).
| Population/Site | Period/Age | Economic mode | VCF Prevalence | Source | |
| Male | Female | ||||
| Dahekou, Yicheng, Shangxi, China | Western Zhou (1045-771 BCE) | Urban | 6.5% (3 of 46) Middle aged man | 24.3% (18 of 74) Young to Middle aged women | This study |
| Xitun, Yanqin, Beijing, China | Han (202BCE-220CE) | Millet Agriculture | 0% (0 of 126) | 0.6% (1 of 167) | Zhou, 2014 |
| Gouwan, Xichuan, Henan, China | Late Neolithic (5000-3000BCE) | Rice and Millet Agriculture | 2.6% (1 of 39) | 0% (0 of 26) | Wang, 2015 |
| Matengkong, Xi’an, Shaan Xi, China | Eastern Zhou (770-256BCE) | Agriculture | 12.5% (3 of 24) | 7.7% (2 of 26) | Wang, 2019 |
| Xuecun, Henan, China | Han (202BCE-220CE) | Agriculture | 2.6% (2 of 76) | 1.6% (1 of 61) | Sun, 2013 |
| Da’an, Jilin, China | Warring States to West Han (475BCE-8CE) | Hunting-Fishing | 2.8% (1 of 36) | 0% (0 of 67) | Xiao, 2014 |
| Lamadong, Beipiao, Liaoning, China | Jin (31-420CE) | Millet Agriculture | 0.5% (1 of 214) | 0% (0 of 190) | Chen, 2009 |
| Changle, Zhongwei, Ningxia, China | Han (202BCE-220CE) | Agriculture | 5.6% (2 of 36) | 10.3% (3 of 29) | Zhang, 2018 |
| Taojiazai,Xining, Qinghai, China | Han-Jin (202BCE-420CE) | Agriculture | 0.6% (1 of 175) | 0% (0 of 167) | Zhang, 2008 |
| Jucun, Jiangxian, Shanxi, China | Western Zhou (1046-771BCE) | Agriculture | 0.8% (2 of 258) | 0.7% (2 of 296) | Zhao, 2018 |
| Gaohong, Liulin, Shanxi, China | Late Shang (1250-1046BCE) | Agriculture | 27.3% (3 of 11) | 0% (0 of 5) | Liang, 2017 |
| Xiaonanzhuang, Taiyuan, Shanxi, China | Warring States (475-221BCE) | Agriculture | 0% (0 of 7) | 10.0% (1 of 10) | Hou, 2017 |
| Niedian, Jinzhong, Shanxi, China | Zhou (1046-256BCE) | Agriculture | 9.1% – 5 (all female) of 55 male & female combined | Hou, 2017 | |
| North Liujiazhuang, Anyang, Henan, China | Late Shang (1250-1046BCE) | Agriculture | 6.1% – 3 (all female) of 49 male & female combine | Yuan, 2010 | |
| Chuanzhang, Zungeer, Inner Mongolia, China | Warring States to Han (475BCE-8CE) | Agriculture | 0% (0 of 165) | 0.8% (1 of 132) | Ana’er, 2018 |
| Liangwangcheng, Peizhou, Jiangsu, China | Late Neolithic (2800-2500BCE) | Rice Agriculture | 1.2% – 1 (sex-age unknown) of 86 male & female combined | Zhu et al., 2013 | |
| Chongpingyuan, Yichuan, Shaanxi, China | Early Spring-Autumn Period (770-650BCE) | Agriculture | 14.3% (1 of 7) | 9.0% (1 of 11) | Chen et al., 2018 |
| Giecz, Poland | 950-1250 CE | Rural | 50.0%, Adults ≥ 18 years | 29.8%, ≥ 18 years | Agnew et al., 2015 |
| Poznań-Sródka, Poland | 950-1250 CE | Urban | 0%, Adult ≥ 18 years | 3.7%, ≥ 18 years | Agnew et al., 2015 |
| Modern American Women | 5-10% middle aged women | Ensrud and Schousboe, 2011 | |||
Agriculture has been one of the most dangerous occupations since its origin due to high physical demands (McCurdy and Carroll 2000), and is still dangerous in modern times with the involvement of machinery (Myers 2001; Chari et al. 2017). Past bioarchaeological studies support the conclusion that rural areas with agricultural practices such as farming and animal husbandry had higher frequencies of trauma than urban areas (Judd and Roberts 1999; Djurić et al. 2006; Agnew et al. 2015). For example, Agnew et al.’s (2015) study of medieval rural and urban settings shows a distinct difference in the prevalence of trunk fractures (including VCF), with VCF prevalence being higher in rural areas than urban areas. Overall, agricultural populations engaged in a laborious lifestyle, reflected in a variety of injuries related to repetitive and high-risk activities. Although urban populations engaged in craft specialization and participated in repetitive activities, their lifestyle resulted in a lesser risk of fracture (Agnew et al. 2015). The lack of skull fractures at Dahekou also suggests a low risk of injury from intentional interpersonal conflicts, while the relatively high incidence of limb and rib bone injuries in males might indicate a lifestyle with higher physical stress. At the rural Medieval Poland Giecz site, the prevalence of VCF was 50%, whereas the prevalence of VCF at the urban Medieval Poland Poznań-Sródka site was close to 0% (Agnew et al. 2015). The prevalence of VCF was 6.5% in males at Dahekou, corroborating the presumption of an urban lifestyle at Dahekou. The relatively low prevalence of VCF suggests that the economic activities males at Dahekou participated in were less stressful than a typical agricultural lifestyle in terms of the stress on the spine, though nutrition and genetic adaptation (hence better bone quality) might be among the biological and environmental factors in addition to sample bias in these studies. Nonetheless, the low prevalence of vertebral fractures corroborates with the presumption of an urban lifestyle for the economic mode of the Dahekou population.
Spinal Osteophytosis and Gender-Based Labor Divisions
At Dahekou, the high limb and rib bone fractures might also suggest that males from the site had higher environmentally specific physical stressors than females (Appendix Table 1), which might indicate a gender-biased division of labor within in an urban lifestyle. On the other hand, it is proposed that females would be more involved in childcare and household chores, as well as the business and service industries (Agnew et al. 2015). However, females from the Dahekou site would have engaged in some physically demanding tasks,such as water transportation, child-carrying, food processing, and needlework; heavy loading in these scenarios would be on the cervical (head-loading) and lumbar (compressive and bending forces) vertebral segments. It is worth noting that there would have been an efficient water supply system during the Zhou Dynasty, in which joined clay pipes equipped with cleaning pools and valve trenches were installed underground to transfer water into the city (An 1992; Du and Chen 2007). In the city of the Ba State, fresh water from water cisterns might have been transferred to households using pots. Women might have used tumplines to carry water home as in other ancient human societies (Bridges 1994), or for other items such as children, materials and products (Moromizato et al. 2007). In addition, there were certainly more factors complicating this gender-divided labor model, yet it formed a simplified economic model for further studies involving other factors. Researchers would need to consider the life history of females (pregnancy, postpartum, weaning, and menopause) and other environmental and socioeconomic parameters.
Osteophytic grades were generally high in the lumbar area for both males and females, as exhibited by the grades on L4 and L5 (Table 8). Akin to VCF, these vertebrae contribute most to the ergonomic support for upright positions and are more prone to compromise. At Dahekou, the high severity of osteoarthritis in males at both the vertebral body and facet joints suggest an arduous (though less dangerous) urban life than rural life.
| Site | Date | Economic Mode | Vertebrae most severely affected by osteophytosis | References |
| Dahekou, China | 1,045 – 771 BCE | Urban | C5-6, T7-L5 in males | This study |
| Joseon People, South Korea | 1,500 – 1,900 CE | Agriculture | C5, T9-10, L4-5 | Kim et al., 2012 |
| Raymond Dart Collection & University of Pretoria Collection, South African | Contemporary skeletal collections derived from cadavers | Mixed contemporary lifestyles | C5, L4-5 | Van der Merwe et al., 2006 |
| Ca-Ala-329, California, U.S.A. | 1,200 –1,600 CE | L4-5 | Jurmain, 1990 | |
| Perry Site Lu 25, Alabama, U.S.A. | 4,000 – 1,000 BCE | Hunter-gatherers | L3-4, C5-6, T7-9 | Rojas-Sepulveda et al., 2008 |
| Koprivno, Croatia | 1,600 – 1,900 CE | Rural Agriculture/Pastoral | C6, T4, L5 | Novak & Slaus, 2011 |
| Sisak, Croatio | 1,600 – 1,900 CE | Urban | C5, T7, L5 | Novak & Slaus,2011 |
| Spitalfields, England | 1,800 – 1,900 CE | Urban | T8-9 | Waldron, 1991 |
| El Mirador cave, Spain | 4,880 – 4,390 BP | Agriculture and animal husbandry | L>T>C | Yustos et al., 2021 |
Joint facet osteoarthritis in the spine is caused by a combination of multiple factors including usage, overload, age-related changes, injury, and possible genetic predisposition. Manifestations of the condition involves pain, swelling, and loss of flexibility (Butler et al. 1990; Dreyfuss et al. 1994; Dreyer and Dreyfuss 1996; Manchikanti et al. 2004; Cavanaugh et al. 2006; Kalichman and Hunter 2007; Gellhorn et al. 2013; Kim et al. 2015). The overall severity of joint facet osteoarthritis at Dahekou was low, yet males had higher development of the condition than females, indicating multiple factors that included overall body mass, higher physical stress, genetic predisposition, or sample bias. Males also exhibited asymmetry in facet joint osteoarthritis [similar to a finding by Bridges (1994) in prehistoric Native Americans], suggesting relatively more lateral bending activities than anterior or posterior bending involved in urban professions (including metallurgy, pottery making, glass working, stone cutting, and brick making), in addition to right-favored handedness. Female patterns of joint facet osteoarthritis were more symmetrical, suggesting more axially oriented movements or a greater relation to the flexion and extension of urban female activities, such as head-loading (i.e., water transportation), childcare, trade, and work in the service industry. It is worth noting that the status of women during the Bronze Age may have been different between China and Europe. For example, archaeological working in southeastern Spain revealed that women could have been correlated to the ruling class in the Bronze Age El Argar society (about 4,000 years ago) (Lull et al. 2021), which would denote a totally different patterns of labor division.
Inequality of Spinal Health at Dahekou
Ultimately, our research demonstrates that there were VCF-related, female-biased health inequalities with a high prevalence of osteoporotic fractures in females at the Dahekou site (an urban setting with a feudal economy). This socioeconomic context was essential in identifying a sex-based health issue within this population. We did uncover a male-biased health inequality in terms of vertebral osteoarthritis. Males experienced more severe cases of the condition, indicating more male-biased and physically demanding socioeconomic roles. As mentioned above, bone loss-induced fractures were uncommon in physically active populations (Jónsson et al. 1992; Sanders et al. 2002; Pisani et al. 2016). Thus, the high osteoarthritis coupled with the low VCF in males at Dahekou (and high prevalence of rib fractures) suggests higher physical stress in males as well. Vice versa, the low osteoarthritis coupled with high VCF in females would suggest that women at Dahekou were generally involved in low labor-intensive activities.
However, even though stress on the trunk from daily activities was higher in males than females, females not only had more VCF but also early onset of VCF. Although we proposed that the Dahekou population was an urban setting based on the overall low male injuries and other indicators, females at Dahekou had a VCF rate at 24.3%, which was similar to the prevalence rate of 29.8% of females in the rural setting of the Giecz site in Medieval Poland. Modern-time vertebral fractures are typically the result of high-energy trauma, such as a fall from height, autmobile accident, sports accident, or gunshot wounds. Nevertheless, why did Dahekou females have such a high frequency of VCF? Could this high rate of vertebral fractures be the outcome from a high incidence of trauma, such as from accidental falls or violence against women? Would this pattern indicate an unrecognized high risk in a localized urban setting at Dahekou, overall poor bone quality in females at Dahekou, or just a sample bias? There were no signs of intensive interpersonal conflict at Dahekou and no evidence of systematic violence against women, as depicted in the high incidence of head injuries and rib fractures in females from the Heartland of the Wari Empire, Peru (Tung 2012); thus, this alternative explanation is less likely. Previous stable isotopic analysis of human bones from the Zhou Dynasty have demonstrated dietary differences favoring males (i.e., Dong et al. 2017; Barbera et al. 2020; Miller et al. 2020), while findings indicating egalitarianism between males and females in access to food resourses have been recovered from some archaeological sites of the Zhou Dynasty as well (Lan 2017; Ling 2010; Ling et al. 2017; Pechenkina 2018). These data indicate the possibility of health inequalities that were linked to a male-biased inequality of food access in ancient China. In our opinion, the possible major causes of VCF in the females of Dahekou were due to hormone deficiency, poor nutrition, and/or lack of physical activities compared to their age-matched male counterparts. The latter conclusion was in accordance with an urban lifestyle, while the former might indicate that females had lower socioeconomic statuses compared to males in ancient societies in China (Holmgren 1981), as has been seen in other ancient complex societies globally (Schepartz et al. 2017) and therefore, had lower diet standards and poorer nutrition than males (i.e., Dong et al. 2017; Barbera et al. 2020; Miller et al. 2020; but see Lan 2017; Ling 2010; Ling et al. 2017; Pechenkina 2018). High prevalence of low back pain is also found in pregnant, postpartum, and osteoporotic females (Katonis et al. 2011; Krishnakumar et al. 2016; Wang et al. 2016). In this regard, females at Dahekou had an overall lower spinal health status.
As earlier mentioned, the Dahekou population provides a useful urban setting: a relatively peaceful and feudalistic society whose production mode fostered various age and work related skeletal pathologies. However, a full body investigation is thus warranted, including study of the skull, trunk, and extremities (along with arthritis of the limb joints).
Nonetheless, the findings of this study demonstrate the disparities of vertebral health between the two sexes. While age-related changes of female vertebral column may be more susceptible to hormone deficiency-related pathophysiologies such as osteoporosis, age-related changes of the male vertebral column may be more prone to heavy, labor induced physical stress. Accordingly, the male-female health inequality found in our study is probably the outcome of combined factors. These factors include sex-based differences in physioanatomy (an image analysis of bone qualities including bone mass, distribution and biomechanical strength is warranted), sex-based stress levels (differences between non-specific stress indicators on the skeletal remains of males and females), gendered divisions of socioeconomic roles in an urban setting, nutrition (an isotopic analysis for diet reconstruction is warranted), and social statuses (a mortuary analysis of grave goods and grave values is warranted). Coupled with imaging, isotropic, and mortuary analyses, knowledge of how sex-based differences in pathophysiology and different levels of physical stress (due to gendered divisions of labor) influence the etiology and pathophysiology of skeletal degenerative diseases can reveal the impact of anatophysiological and sociocultural factors on health disparities in bioarcheological studies.
Limitations of This Study and Future Research Directions
In this study, the model involving sex-based differences in anatophysiology and gender-based divisions of labor between males and females was simplified. Additional factors such as overall skeletal health/pathology status, bone quality analysis (total bone mass, patterns of bone distribution, and bone biomechanical strength) should be considered. A dietary analysis along with grave goods and burial values should be included as well. For example, it is not possible at the current stage to confirm poor nutrition and lack of physical activity in females at the Dahekou site. The age group division was arbitrary and used as a convenient method to generate sex-age groups comparable in males and females. However, the life history of females (i.e., pregnancy, breastfeeding, a weaning, fertility, and menopause) should be taken into consideration in the future.
The excavation at Dahekou continues. An expanded study to have all skeletons studied, especially those from elite classes (with larger grave chambers and richer grave goods), would be helpful in studying the impact of social stratification (Zhou et al. 2021). For example, an investigation of females of different social classes during the Bronze Age using the life course approach following Agarwal (2016) would be suitable. Moreover, radiological analysis of vertebrae would also assist in better characterizing bone changes in aging spines. Furthermore, we suggest that spine pathology would be better studied within the context of the whole skeleton.
There has been limited information on facet joint osteoarthritis occuring after the degeneration of the intervertebral disc (Butler et al. 1990). This suggests that facet joint diseases are secondary to mechanical changes in the overall loading patterns of the spine. However, due to differences in sample sizes, a paired study of osteophytosis in vertebral body and joint facets was not carried out in the current investigation. This process would be possible in the future when more skeletal remains at Dahekou are available.
The term degenerative joint disease (DJD) was used in this study, though there are arguments that they are age-related adaptations (Anderson and Loeser 2011; Yustos et al. 2021). The scoring grades were still rough; for example, the readings for joint facet osteoarthritis,relied more on the development of osteophytes and mean scores of osteophytosis. For the joint facet investigation, the number of individuals suited for this section of our study was imbalanced (71 females vs. 22 males). However, because our study was the first systematic investigation of facet joint osteoarthritis in skeletons from Northern China, the results were reported to prompt more studies in this direction. For the data analysis, mean values of osteophytosis were averages of two or four readings in a vertebra, which might affect the severity of osteoarthritis. All of these results and analyses need to be refined in the future. The reported results focused on trends, with statistical significance in differences, which was due to the small or biased sample sizes and relatively high Coefficient of Variations (for example, the scores of osteophytosis in vertebral body was 1.22 for all specimens combined), which lowered confidence in the interpretation of our results.
In addition, three vertebral segments in this study (cervical, thoracic, and lumbar) were investigated to assess the development of osteophytosis and prevalence of VCF. From the one-hundred and twenty individuals in the Dahekou population included in this study, there were 2578 total vertebrae (Appendix Table 1). On average, 21.5 vertebrae out of 24 (89.6%) in these three spinal segments combined were in good condition, and therefore, included in this study. Thus, there is a need tobe cautious of sampling bias and exaggeration of difference compared to other sites with less favorable preservation status (Tables 7–8).
Conclusion
Vertebral compressive fractures, osteophytosis of the vertebral body, and joint facet arthritis were investigated in skeletal remains from the Bronze Age Dahekou site in China. Patterns of VCF and DJD in different sex-age groups indicated a sex-based difference in paleoepidemiology, mostly likely due to differences in pathophysiological and socioeconomic roles during a peaceful and feudalistic urban economy. Overall, females had a higher prevalence of VCF, with the majority of cases found in females between twenty-five and thirty years old. This result suggests increased cases of osteoporosis in females, a physiological disease that affected more females. The prevalence of osteophytosis of the vertebral body was higher in the more senior group for both males and females, signifying natural development with skeletal use along with age-related changes. Despite the knowledge that age was a factor, there was also a sex-based difference, as the overall severity of osteophytosis was higher in males than in females. This finding might demonstrate the consequences of gender-based labor divisions or sex-based genetic dispositions. Likewise, males had more severe joint facet arthritis than females, indicating higher physical stress and lower spinal health status in males. These patterns of DJD in different sex-age groups point towards a sex-based difference in paleoepidemiology, suggesting the combined effect of gendered differences in life history and socioeconomic roles. Hence, these findings signify a sex-based health disparity. Comparable to other groups with similar socio-economic structures, the Dahekou population lived in a feudalistic urban area during a peaceful time. A comprehensive study is warranted to ascertain whether city-state Ba exemplifies ancient Chinese life during the Bronze age. Knowledge of how sex-based differences in pathophysiology, as well as different levels of physical stress (due to gendered divisions of labor) influence the etiology and pathophysiology of skeletal degenerative diseases is beneficial. This view will help distinguish these elements from other sociocultural factors contributing to health disparities in historic and contemporary environments.
Acknowledgement
We thank the Shanxi Provincial Institute of Archaeology for providing materials for this research. Our gratitude to Ms. Meghann Holt for editing the English. We are also grateful to Dr. Li Sun for help and support of various kinds. We also thank the editor and reviewers for their constructive comments.
Conflict of interest
None.
Contributions from individual authors
T.H.: Data collection and analyses. W.Z., H.Z.: Data collection. Y.X.: Specimen accumulation and curation. X.Z.: Data analysis and statistics; Q.Z.: Project conception and data analysis. Q.W.: Project conception, data collection, data analysis, writing manuscript.
Data availability statement
Research data and images will be available in the public domain after the completion and publication of the study’s findings. Entities include Jilin University and Texas A&M University.
T.H. was supported by the Youth Project of the National Philosophy and Social Sciences Foundation (Grant No. 21CKG024), which covered the research component of this study. Y.T.X. was supported by the Chinese National Social Science Major Projects Fund (Grant No. 17ZDA218), which covered the excavation and curation of the specimens used in this study. Q.W. was supported by the Texas A&M University T3 Grant, which involved the research component of this study.
References
Adams MA, Dolan P. 2012. Intervertebral disc degeneration: Evidence for two distinct phenotypes. Journal of Anatomy 221:497–506.
Agarwal S. 2016. Bone morphologies and histories: Life course approaches in bioarchaeology. The Yearbook of Physical Anthropology 159 (S61):130–149.
Agnew AM, Betsinger TK, Justus HM. 2015. Post-Cranial Traumatic Injury Patterns in Two Medieval Polish Populations: The Effects of Lifestyle Differences. PLoS One 10(6):e0129458.
Aguirre MFI, Tsirikos AI, Clarke A. 2020. Spinal injuries in the elderly population. Orthopaedics and Trauma 34 (5):272–277.
An J. 1992. Zhongguo Kaogu (China Archeology). Shanghai: Shanghai Guji Chubanshe (Shanghai Ancient Literature Publishing House).
Ana’er. 2018. A research on the human skeletons of Chuanzhang site, Zhungeer County, in Inner Mongolia. M.S. Thesis. Changchun, China: Jilin University.
Anderson AS, Loeser RF. 2011. Why is osteoarthritis an age-related Disease? Best Practice & Research: Clinical Rheumatology 24(1):15–26.
Barbera AR, Pechenkina K, Miller M, Halcrow S, Fan W. 2020. Women of Zhenghan: Documenting gender inequality based on skeletal assemblages from Eastern Zhou China. American Journal of Physical Anthropology 171:237–238.
Bailey A. 2009. Risk factors for low back pain in women: Still more questions to be answered. Menopause 16:3–4.
Barford P. 2005. Silent centuries: the society and economy of the northwestern Slavs. In: Curta F (ed). East Central and Eastern Europe in the Early Middle Ages. University of Michigan Press: Ann Arbor, p. 60–102.
Benoist M. 2003. Natural history of the ageing spine. European Spine Journal 12 (Suppl 2): S86–S89.
Braveman P. 2006. Health disparities and health equity: Concepts and measurement. Annual Review of Public Health 27:167–194.
Brickley M. 2002. An investigation of historical and archaeological evidence for age related bone loss and osteoporosis. International Journal of Osteoarchaeology 12:364–371.
Bridges PS. 1994. Vertebral arthritis and physical activities in the Prehistoric Southeastern United States. American Journal of Physical Anthropology 99:83–93.
Buikstra JE, Ubelaker DH. 1994. Standards for data collection from human skeletal remains. (No.44, 1–272). Fayetteville: Arkansas Archeological Survey Research Series.
Burrell L, Maas M, van Gerven D. 1986. Patterns of long-bone fractures in two Nubian cemeteries. Human Evolution 1: 495–506.
Butler D, Trafimow JH, Andersson GBJ, Mcneill TW, Huckman MS. 1990. Discs Degenerate before facets. Spine 15(2):111–113.
Cavanaugh JM, Lu Y, Chen C, Kallakuri S. 2006. Pain generation in lumbar and cervical facet joints. Journal of Bone and Joint Surgery American volume 88 (Suppl 2): 63–67.
Chari R, Kress AM, Madrigano J. 2017. Injury and Illness Surveillance of U.S. Agricultural Workers: Assessment of Recommendations and Actions. Santa Monica, CA: RAND Corporation.
Chapman FH. 1972. Vertebral osteophytosis in prehistoric populations of central and southern Mexico. American Journal of Physical Anthropology 36:31–38.
Chen L, Ding Y, Xiong J, Li Y. 2018. A study on the human skeleton remains unearthed from the Chongpingyuan site in Yichuan County, Shaanxi. Archaeology and Cultural Relics 2:118–128.
Chen S. 2009. A research on the human skeletons of Sanyan culture from Lamadong graveyard. Ph.D. Thesis. Changchun, China: Jilin University.
Cooper C, Atkinson E, O’Fallon W, Melton L. 1992. Incidence of clinically diagnosed vertebral fractures: A population-based study in Rochester, Minnesota 1985–1989. Journal of Bone and Mineral Research 7:221–227.
Cummings SR, Black DM, Rubin SM. 1989. Lifetime risks of hip, Colles’, or vertebral fracture and coronary heart disease among white postmenopausal women. Archives of Internal Medicine 149:2445–2448.
Cummings S, Kelsey J, Nevitt M, O’Dowd K. 1985. Epidemiology of osteoporosis and osteoporotic fractures. Epidemiology Review 7:178–208.
Cummings SR, Melton LJ. 2002. Epidemiology and outcomes of osteoporotic fractures. The Lancet 359:1761–1767.
Djurić MP, Roberts CA, Rakočević ZB, Djonić D, Lešić AR. 2006. Fractures in late medieval skeletal populations from Serbia. American Journal of Physical Anthropology 130:167–178.
Dong Y, Morgan C, Chinenov Y, Zhou L, Fan W, Ma X, Pechenkina K. 2017. Shifting diets and the rise of male-biased inequality on the Central Plains of China during Eastern Zhou. Proceedings of the National Academy of Sciences of the United States of America 114:932–937.
Dreyer SJ, Dreyfuss PH. 1996. Low back pain and the zygapophysial (facet) joints. Archives of Physical Medicine and Rehabilitation 77:290–300.
Dreyfuss P, Tibiletti C, Dreyer SJ. 1994. Thoracic zygapophyseal joint pain patterns. A study in normal volunteers. Spine 19:807–811.
Du P, Chen H. 2007. Water supply of the cities in ancient China. Water Science & Technology: Water Supply 7(1):173–181.
Ensrud KE, Schousboe JT. 2011. Clinical practice. Vertebral fractures. The New England Journal of Medicine 364(17):1634–1642.
Feng T. 2014. Society of Imperial Power: Reinterpreting China’s “Feudal Society”. Journal of Chinese Humanities 1:25–50.
Fillingim RB. 2003. Sex, gender and pain: The biopsychosocial model in action XX vs. XY. The International Journal of Sex Differences in the Study of Health, Diseases and Aging 1:98–101.
Fraser RD, Bleael JF, Moskowitz RW. 1997. Spinal degeneration. Pathogenesis and medical management. In: Frymoyer JW (ed). The adult spine: principles and practice. Lippincott Raven, Philadelphia, pp. 735–758.
Geber J. 2015. Comparative study of perimortem weapon trauma in two early medieval skeletal populations (AD 400–1200) from Ireland. International Journal of Osteoarchaeology 25:253–264.
Gellhorn AC, Katz JN, Suri P. 2013. Osteoarthritis of the spine: The facet joints. Nature Reviews Rheumatology 9(4):216–224.
Grauer AL, Roberts C. 1996. Paleoepidemiology, healing, and possible treatment of trauma in the medieval cemetery population of St. Helen-on-the-Walls, York, England. American Journal of Physical Anthropology 100:531–544.
Guo L. 2015. Preliminary study of human skeletal remains from the Dahekou cemetery, Yicheng (2009–2011). M.S. Thesis. Changchun, China: Jilin University.
Han T. 2019. A research on the human skeletal remains from Dahekou graveyard in Yicheng, Shanxi. Ph.D. Thesis. Changchun, China: Jilin University.
Holmgren J. 1981. Myth, fantasy or scholarship: Images of the status of women in traditional China. The Australian Journal of Chinese Affairs 1981(6):147–170.
Hou K. 2017. The research on the human skeletons from the Pre-Qin Period tombs excavated in the College Town in Yuci, Shanxi. Ph.D. Thesis. Changchun, China: Jilin University.
Hou K, Wang M, Zhu H. 2017. Bioarcaheological research of diseases in human vertebrae from Xinglonggou Site, Chifeng City, Inner Mongolia. Acta Anthropologica Sinica 36:87–100.
Jónsson B, Gardsell P, Johnell O, Redlund-Johnell I, Sernbo I. 1992. Differences in fracture patterns between an urban and rural population: A comparative population-based study in southern Sweden. Osteoporosis International 2:269–273.
Judd MA, Roberts CA. 1999. Fracture trauma in a medieval British farming village. Am J Phys Anthropol 109:229–243.
Jurmain R. 1990. Paleoepidemiology of a central California prehistoric population from Ca-Ala-329: II. Degenerative disease. American Journal of Physical Anthropology 83:83–94.
Kaidonis JK. 2008. Tooth wear: The view of the anthropologist. Clinical Oral Investigations 12 (Suppl 1), 21–26.
Kalichman L, Hunter DJ. 2007. Lumbar facet joint osteoarthritis: A review. Seminars in Arthritis and Rheumatism 37:69–80.
Katonis P, Kampouroglou A, Aggelopoulos A, Kakavelakis K, Lykoudis S, Makrigiannakis A, Alpantaki K. 2011. Pregnancy-related low back pain. Hippokratia 15(3):205–210.
Katzman WB, Wanek L, Shepherd JA, Sellmeyer DE. 2010. Age-related hyperkyphosis: Its causes, consequences, and management. Journal of Orthopaedic & Sports Physical Therapy 40(6):352–360.
Kilgore L, Jurmain R, Van Gerven D. 1997. Palaeoepidemiological patterns of trauma in a medieval Nubian skeletal population. International Journal of Osteoarchaeology 7:103–114.
Kim DK, Kim MJ, Kim YS, Oh CS, Shin DH. 2012. Vertebral osteophyte of pre-modern Korean skeletons from Joseon tombs. Anatomy & Cell Biology 45:274–281.
Kim J, Ali MH, Wydra F, Li X, Hamilton JL, An HS, Cs-Szabo G Andrews S, Moric M, Xiao G, Wang JH-C, Chen D, Cavanaugh JM, Im H-J. 2015. Characterization of degenerative human facet joints and facet joint capsular tissues. Osteoarthritis Cartilage 23:2242–2251.
Knüsel CJ, Göggel S, Lucy D. 1997. Comparative degenerative joint disease of the vertebral column in the medieval monastic cemetery of the Gilbertine priory of St. Andrew, Fishergate, York, England. American Journal of Physical Anthropology 103:481–495.
Krishnakumar R, Kumar AT, Kuzhimattam MJ. 2016. Spinal compression fractures due to pregnancy-associated osteoporosis. Journal of Craniovertebral Junction & Spine 7(4):224–227.
Lan D. 2017. Isotope analysis on human and animal’s bone unearthed from Zaoshugounao Site in Chun Hua County, Shaanxi Province. MA thesis, Northwest University (in Chinese).
Leveille SG, Zhang Y, McMullen W, Kelly-Hayes M, Felson DT. 2005. Sex differences in musculoskeletal pain in older adults. Pain 116:332–338.
Li L, Zhu L, Xie Y. 2021. Proteomics analysis of the soil textile imprints from tomb M6043 of the Dahekou Cemetery site in Yicheng County, Shanxi Province, China. Archaeological and Anthropological Sciences 13, 7.
Liang N. 2017. A research on the human skeletons from Gaohong graveyard in Liulin of Shanxi Province. Ph.D. Thesis. Changchun, China: Jilin University.
Ling X. 2010. Study on dietary of ancient Qin people. Ph.D. dissertation, Northwest University (in Chinese).
Ling X, Chen X, Sun BJ., Zhang TE, Chen L, Zhao CC. 2017. Stable Isotopic analysis on human bones on later western Zhou period excavated from the Rui State Cemetery at Liangdai Village in Hancheng City. Western China Archaeology (in Chinese) 2:249–258.
Lovejoy CO. 1985. Dental wear in the Libben population: its functional pattern and role in the determination of adult skeletal age at death. American Journal of Physical Anthropology 68:47–56.
Lovejoy CO, Meindl RS, Pryzbeck TR, Mensforth RP. 1985. Chronological metamorphosis of the auricular surface of the ilium: A new method for the determination of adult skeletal age at death. American Journal of Physical Anthropology 68:15–28.
Lovell NC. 1994. Spinal arthritis and physical stress at Bronze Age Harappa. American Journal of Physical Anthropology 93:149–164.
Lull V, Rihuete Herrada C, Risch R, Bonora B, Celdrán-Beltrán E, Fregeiro MI, Molero C, Moreno A, Velasco-Felipe C, Andúgar Martínez L, Haak W, Villalba-Mouco V, Micó Pérez R, & Oliart Caravatti C. 2021. Emblems and spaces of power during the Argaric Bronze Age at La Almoloya, Murcia. Antiquity 95(380):329–348.
Manchikanti L, Boswell MV, Singh V, Pampati V Damron KS, Beyer CD. 2004. Prevalence of facet joint pain in chronic spinal pain of cervical, thoracic, and lumbar regions. BMC Musculoskeletal Disorder 5:15.
McCurdy S, Carroll D. 2000. Agricultural injury. American Journal of Industrial Medicine 38:463–480.
Merbs CF. 1996. Spondylolysis and spondylolisthesis: a cost of being an erect biped or a clever adaptation? The Yearbook of Physical Anthropology 39:201–228.
Miller MJ, Dong Y, Pechenkina K, Fan W, Halcrow S. 2020. Dietary practices over the life-course: Gender and food in two urban Eastern Zhou communities (ancient Zhenghan City, China). American Journal of Physical Anthropology 171:188–189.
Miller JA, Schmatz C, Schultz AB. 1988. Lumbar disc degeneration: Correlation with age, sex, and spine level in 600 autopsy specimens. Spine (Phila Pa 1976) 13:173–178.
Missikpode C, Peek-Asa C, Young T, Swanton A, Leinenkugel K, Torner J. 2015. Trends in non-fatal agricultural injuries requiring trauma care. Injury Epidemiology 2(1):30.
Moromizato K, Fukumine T, Doi N, Hanihara T, Nishime A, Yoneda M, Ishida H. 2007. Degenerative Diseases of the Spines of Early Modern Human Remains from Kumejima, Okinawa. Anthropological Science 115:25–36.
Molnar S. 1972. Tooth wear and culture: A survey of tooth functions among some prehistoric populations. Current Anthropology 13:511–526.
Myers J. 2001. Injuries Among Farm Workers in the United States 1995. National Institute for Occupational Safety and Health: Cincinnati.
Novak M, Slaus M. 2011. Vertebral pathologies in two early modern period (16th-19th century) populations from Croatia. American Journal of Physical Anthropology 145:270–281.
Ostrowska A. 2012. Health inequalities-gender perspective. Przegl Lek 69(2):61–66.
Pechenkina K. 2018. Of millets and Wheat: Diet and health on the Central Plain of China during the Neolithic and Bronze Age. In: Goldin PR (ed) Handbook of Early Chinese History. London: Routledge, p. 39–60.
Papadakis M, Sapkas G, Papadopoulos EC, Katonis P. 2011. Pathophysiology and biomechanics of the ageing spine. The Open Orthopaedics Journal 5:335–342.
Pili R, Gaviano L, Pili L, Petretto DR. 2018. Ageing, Disability, and Spinal Cord Injury: Some Issues of Analysis. Current Gerontology and Geriatrics Research. Article ID 4017858.
Pisani P, Renna MD, Conversano F, Casciaro E, Di Paola M, Quarta E, Muratore M, Casciaro S. 2016. Major osteoporotic fragility fractures: Risk factor updates and societal impact. World Journal of Orthopedics 7(3):171–181.
Riggs BL, Wahner HW, Melton LJ, 3rd, Richelson LS, Judd HL, Offord KP. 1986. Rates of bone loss in the appendicular and axial skeletons of women. Evidence of substantial vertebral bone loss before menopause. Journal of Clinical Investigation 77:1487–1491.
Rojas-Sepúlveda C, Ardagna Y, Dutour O. 2008. Paleoepidemiology of vertebral degenerative disease in a Pre-Columbian Muisca series from Colombia. American Journal of Physical Anthropology 135:416–430.
Sanders KM, Nicholson GC, Ugoni AM, Seeman E, Pasco JA, Kotowicz MA. 2002. Fracture rates lower in rural than urban communities: the Geelong Osteoporosis Study. Journal of Epidemiology & Community Health 56:466–470.
Schepartz LA, Stocker SR, Davis JL, Papathanasiou A, Miller-Antonio S, Murphy JMA, et al. 2017. Mycenaean Hierarchy and Gender Roles: Diet and Health Inequalities in Late Bronze Age Pylos, Greece. In: Klaus HD, Harvey AM, Cohen MA (eds). Bones of Complexity: Bioarchaeological Case Studies of Social Organization and Skeletal Biology. Gainesville, FL: University Press of Florida, p. 141–172.
Shimoda Y, Nagaoka T, Moromizato K, Sunagawa M, Hanihara T, Yoneda M, et al. 2012. Degenerative changes of the spine in people from prehistoric Okhotsk culture and two ancient human groups from Kanto and Okinawa, Japan. Anthropological Science 120:1–21.
Snodgrass JJ. 2004. Sex differences and ageing of the vertebral column. Journal of Forensic Sciences 49(3):458–463.
Sofaer-Derevenski JR. 2000. Sex differences in activity-related osseous change in the spine and the gendered division of labor at Ensay and Wharram Percy, UK. American Journal of Physical Anthropology 111:333–354.
Steckel RH, Larsen CS, Roberts CA, Baten J. 2019. The Backbone of Europe: Health, Diet, Work and Violence over Two Millennia. Cambridge: Cambridge University Press.
Steckel RH, Rose JC. 2002. The Backbone of History: Health and Nutrition in the Western Hemisphere. Cambridge: Cambridge University Press.
Stewart TD. 1958. The rate of development of vertebral osteoarthritis in American whites and its significance in skeletal age identification. Leech 28(3–5):144–151.
Sun L. 2013. An anthropological study of the skeletons in tombs of Han, Tang and Song Dynasties from Xuecun site of Xingyang and other sites of Xinzheng, Zhengzhou city, Henan province. Ph.D. Thesis. Changchun, China: Jilin University.
Tung TA. 2012. Violence against Women: Differential Treatment of Local and Foreign Females in the Heartland of the Wari Empire, Peru. In: Martin DL, Harrod RP, Perez VR (eds). The Bioarchaeology of Violence. Gainesville, FL: University Press of Florida, p. 180–198.
Van der Merwe AE, Işcan MY, Lábbè EN. 2006. The pattern of vertebral osteophyte development in a South African population. Int Journal of Osteoarcheology 16:459–464.
Waldron T. 1991. The prevalence of, and the relationship between some spinal diseases in a human skeletal population from London. Int Journal of Osteoarchaeology 1:103–110.
Wamala S, Lynch J. 2002. Gender and socioeconomic inequalities in health. Lund, Sweden: Studentlitteratur.
Wang Y. 2015. A research of the Neolithic human skeleton from Gouwan. M.S. Thesis. Changchun, China: Jilin University.
Wang Y. 2019. The research on the human skeletal remains from Eastern Zhou period tombs excavated in the Matengkong site in Xi’an Shanxi. Ph.D. Thesis. Changchun, China: Jilin University.
Wang Y, Wang J, Kaplar Z. 2016. Increased low back pain prevalence in females than in males after menopause age: evidences based on synthetic literature review. Quantitative Imaging in Medicine and Surgery 6:199–206.
Xiao X. 2014. A research on the human skeletons of Houtaomuga site, Daan City, Jilin Province. Ph.D. Thesis. Changchun, China: Jilin University.
Xie Y, Wang J, Yang J, Li Y, Li J. 2011. The West Zhou Dynasty cemetery at Dahekou, Jichen County, Shanxi Province. Archaeology 7:9–18.
Yuan H. 2010. A research on the skeletons of the medium and small tombs from Yinxu site, Anyang city, Henan province. Ph.D. Thesis. Changchun, China: Jilin University.
Yustos M, Lozano M, Morales JI, Iglesias-Bexiga J, Vergès JM. 2021. Degenerative joint disease in the Chalcolithic population of El Mirador cave (Sierra de Atapuerca, Spain): The vertebral column. International Journal of Osteoarchaeology 31(2):162–175.
Zhang J. 2008. The research on the human skeletons of Han and Jin Dynasties from Taojiazhai graveyard in Xining City of Qinghai Province. Ph.D. Thesis. Changchun, China: Jilin University.
Zhang Q. 2018. A research on the human skeletal remains from Changle graveyard in Zhongwei, Ningxia. Ph.D. Thesis. Changchun, China: Jilin University.
Zhang W, Zhang Q, McSweeney K, Han T, Man X, Yang S, et al. 2021. Violence in the early Iron Age Eurasian Steppe: Cranial trauma in three Turpan Basin populations from Xinjiang, China. American Journal of Physical Anthropology 175:81–94.
Zhao H. 2018. A research on the human skeletons from Jucun graveyard in Jiang County of Shanxi Province. M.S. Thesis. Changchun, China: Jilin University.
Zhou Y. 2014. A research on human skeletons of Xitun graveyard, Yanqing County, Beijing City. Ph.D. Thesis. Changchun, China: Jilin University.
Zhou Y, Fu R, Zheng L, Yan F, Wang Q. 2021. Social Stratification during the Eastern Zhou Dynasty of China (771-476 BCE) - Mortuary and Stable Isotopic Analyses of the Shangshihe Cemetery. International Journal of Osteoarchaeology 31:1001–1029.
Zhu X, Lin L, Zhu H. 2013. A study on the bones unearthed from a Dawenkou culture tomb at the Liangwangcheng site in Pizhou, Jiangsu Province. Southeast Culture 4:53–64.
Zukowski LA, Falsetti AB, Tillman MD. 2012. The influence of sex, age and BMI on the degeneration of the lumbar spine. Journal of Anatomy 220:57–66.
| Individual | Sex | Age | Trauma/Fractures |
| M9161 | F | 30-35 | Right fibular |
| M12199 | M | 25-30 | 6 left 2 right ribs |
| M12170 | M | 30-35 | Nasal bone |
| M9045 | M | 35± | 1 right rib |
| M9236 | M | 35± | Left ulnar distal |
| M12084 | M | 35± | 2 left ribs |
| M12115 | M | 35± | Left ulnar, right clavicle, 2 ribs |
| M9351 | M | 36-40 | 3 (? Left) ribs |
| M10070 | M | 36-40 | Right scapular, 1 left rib |
| M11201 | M | 36-40 | 1 left rib |
| M12004 | M | 36-40 | Right humerus, left 3rd metacarpal |
| M12100 | M | 36-40 | Left tibia two fractures, left radius distal |
| M12127 | M | 36-40 | Right ulnar, 1 left rib |
| M11203 | M | 40± | 2 right ribs |
| M12003 | M | 40± | Mandible right condyle, right & left ribs |
| M12118 | M | 40± | 2 left ribs |
| M12076 | M | 40-45 | 2 right ribs |
| Cervical | Thoracic | Lumbar | Sum | ||||||
| Male | Female | Male | Female | Male | Female | Male | Female | Combined | |
| Adult Young | 117 (6.2) | 244 (5.8) | 208 (10.9) | 454 (10.8) | 82 (4.3) | 198 (4.7) | 407 (21.4) | 896 (21.3) | 1303 (21.4) |
| Adult Middle | 165 (6.1) | 192 (6.0) | 289 (10.7) | 347 (10.8) | 134 (5.0) | 148 (4.6) | 588 (21.8) | 687 (21.5) | 1275 (21.6) |
| Total | 282 (6.1) | 436 (5.9) | 497 (10.8) | 801 (10.8) | 216 (4.7) | 346 (4.7) | 995 (21.6) | 1583 (21.4) | 2578 (21.5) |
| Combined | 718 (6.0) | 1298 (10.8) | 562 (4.7) | 2578 (21.5) | |||||
| Individual | Sex | Age (Years) | Location | VCF Type | |
| Female Adult Young | M10081 | F | 25± | L4-5 | Crush |
| M12108 | F | 25-30 | L5 | Wedge | |
| M7013 | F | 25-30 | T4 | Wedge | |
| M8010 | F | 25-30 | L3-4 | Wedge | |
| M8265 | F | 25-30 | L5 | Crush | |
| M9056 | F | 25-30 | L5 | Crush | |
| M9161 | F | 30-35 | L1, L5 | Crush | |
| Female Adult Middle | M12128 | F | 35-40 | L5 | Crush |
| M9234 | F | 35-40 | L5 | Crush | |
| M9374 | F | 35-40 | T8 | Crush | |
| M9052 | F | 40± | L4-5 | Wedge | |
| M9054 | F | 40± | C5-6 | Crush | |
| M9057 | F | 40± | L5 | Wedge | |
| M7103 | F | 40-45 | L5 | Wedge | |
| M12057 | F | 45-50 | L4-5 (W); L2-3 (beginning of CR) | Wedge & Crush | |
| M9066 | F | 45-50 | T8, L2-3 (CR); T12, L1, L5 (W) | Crush & Wedge | |
| M9163 | F | 45-50 | T7, L1(W); L5 (CR) | Wedge & Crush | |
| M9380 | F | 45-50 | L1 (CR); L2, L5 (W) | Crush & Wedge | |
| Male Adult Middle | M12127 | M | 35-40 | L1 | Crush |
| M12143 | M | 35-40 | T10-12 | Wedge | |
| M12288 | M | 45± | L5 | Crush |
| Adult Young | Adult Middle | |||||||||||
| Male | Female | Male | Female | |||||||||
| N | ≥G2 | % | N | ≥G2 | % | N | ≥G2 | % | N | ≥G2 | % | |
| C1 | 14 | 1 | 7.1 | 35 | 0 | 0 | 24 | 1 | 4.2 | 28 | 0 | 0 |
| C2 | 17 | 2 | 11.8 | 33 | 0 | 0 | 24 | 3 | 12.5 | 31 | 0 | 0 |
| C3 | 16 | 1 | 6.3 | 31 | 1 | 3.2 | 23 | 6 | 26.1 | 27 | 1 | 3.7 |
| C4 | 17 | 2 | 11.8 | 34 | 4 | 11.8 | 22 | 4 | 18.2 | 24 | 5 | 20.8 |
| C5 | 18 | 8 | 44.4 | 36 | 9 | 25.0 | 23 | 8 | 34.8 | 27 | 11 | 40.7 |
| C6 | 18 | 6 | 33.3 | 36 | 8 | 22.2 | 24 | 10 | 41.7 | 27 | 11 | 40.7 |
| C7 | 17 | 6 | 35.3 | 39 | 3 | 7.7 | 25 | 8 | 32.0 | 28 | 3 | 10.7 |
| T1 | 18 | 1 | 5.6 | 39 | 0 | 0 | 25 | 5 | 20.0 | 30 | 0 | 0 |
| T2 | 18 | 1 | 5.6 | 38 | 0 | 0 | 25 | 3 | 12.0 | 29 | 0 | 0 |
| T3 | 17 | 2 | 11.8 | 39 | 0 | 0 | 24 | 5 | 20.8 | 28 | 0 | 0 |
| T4 | 15 | 0 | 0 | 38 | 2 | 5.3 | 24 | 6 | 25.0 | 27 | 0 | 0 |
| T5 | 17 | 1 | 5.9 | 39 | 1 | 2.6 | 23 | 5 | 21.7 | 27 | 1 | 3.7 |
| T6 | 17 | 3 | 17.6 | 37 | 0 | 0 | 23 | 4 | 17.4 | 28 | 4 | 14.3 |
| T7 | 17 | 4 | 23.5 | 36 | 0 | 0 | 22 | 7 | 31.8 | 28 | 5 | 17.9 |
| T8 | 17 | 5 | 29.4 | 37 | 2 | 5.4 | 23 | 6 | 26.1 | 30 | 6 | 20.0 |
| T9 | 17 | 4 | 23.5 | 38 | 3 | 7.9 | 25 | 10 | 40.0 | 30 | 6 | 20.0 |
| T10 | 18 | 5 | 27.8 | 36 | 4 | 11.1 | 25 | 11 | 44.0 | 30 | 5 | 16.7 |
| T11 | 19 | 6 | 31.6 | 39 | 2 | 5.1 | 25 | 9 | 36.0 | 30 | 3 | 10.0 |
| T12 | 18 | 3 | 16.7 | 38 | 0 | 0 | 25 | 6 | 24.0 | 30 | 3 | 10.0 |
| L1 | 16 | 4 | 25.0 | 39 | 1 | 2.6 | 27 | 13 | 48.1 | 30 | 10 | 33.3 |
| L2 | 17 | 3 | 17.6 | 40 | 9 | 22.5 | 26 | 17 | 65.4 | 30 | 13 | 43.3 |
| L3 | 16 | 7 | 43.8 | 39 | 13 | 33.3 | 27 | 19 | 70.4 | 30 | 13 | 43.3 |
| L4 | 17 | 7 | 41.2 | 40 | 17 | 42.5 | 27 | 21 | 77.8 | 29 | 17 | 58.6 |
| L5 | 16 | 7 | 43.8 | 40 | 15 | 37.5 | 27 | 17 | 63.0 | 29 | 18 | 62.1 |
| TOTAL | 407 | 89 | 21.9 | 896 | 94 | 10.5 | 588 | 204 | 34.7 | 687 | 135 | 19.7 |
| Adult Young | Adult Middle | |||||||||||
| Male | Female | Male | Female | |||||||||
| N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | |
| C1 | 14 | 0.14 | 0.53 | 35 | 0.00 | 0.00 | 24 | 0.25 | 0.68 | 28 | 0.00 | 0.00 |
| C2 | 17 | 0.41 | 0.87 | 33 | 0.03 | 0.17 | 24 | 0.50 | 1.18 | 31 | 0.00 | 0.00 |
| C3 | 16 | 0.31 | 0.79 | 31 | 0.23 | 0.62 | 23 | 0.87 | 1.49 | 27 | 0.41 | 0.69 |
| C4 | 17 | 0.47 | 0.87 | 34 | 0.56 | 0.86 | 22 | 0.82 | 1.37 | 24 | 0.71 | 1.00 |
| C5 | 18 | 1.06 | 1.26 | 36 | 0.69 | 0.98 | 23 | 1.26 | 1.36 | 27 | 1.26 | 1.20 |
| C6 | 18 | 1.00 | 1.08 | 36 | 0.72 | 0.94 | 24 | 1.42 | 1.21 | 27 | 1.11 | 1.09 |
| C7 | 17 | 0.88 | 1.05 | 39 | 0.38 | 0.71 | 25 | 1.00 | 1.22 | 28 | 0.54 | 0.69 |
| T1 | 18 | 0.39 | 0.61 | 39 | 0.10 | 0.31 | 25 | 0.56 | 0.82 | 30 | 0.07 | 0.25 |
| T2 | 18 | 0.33 | 0.59 | 38 | 0.08 | 0.27 | 25 | 0.40 | 0.71 | 29 | 0.03 | 0.19 |
| T3 | 17 | 0.53 | 0.72 | 39 | 0.21 | 0.41 | 24 | 0.88 | 1.15 | 28 | 0.21 | 0.42 |
| T4 | 15 | 0.60 | 0.51 | 38 | 0.47 | 0.60 | 24 | 1.04 | 1.12 | 27 | 0.37 | 0.49 |
| T5 | 17 | 0.76 | 0.56 | 39 | 0.59 | 0.55 | 23 | 0.91 | 0.73 | 27 | 0.44 | 0.58 |
| T6 | 17 | 0.94 | 0.83 | 37 | 0.57 | 0.50 | 23 | 1.00 | 0.85 | 28 | 0.57 | 0.74 |
| T7 | 17 | 0.94 | 0.90 | 36 | 0.56 | 0.50 | 22 | 1.23 | 0.97 | 28 | 0.82 | 0.72 |
| T8 | 17 | 0.88 | 0.86 | 37 | 0.62 | 0.59 | 23 | 1.17 | 1.03 | 30 | 0.80 | 0.85 |
| T9 | 17 | 1.18 | 0.88 | 38 | 0.66 | 0.63 | 25 | 1.36 | 1.22 | 30 | 0.73 | 0.78 |
| T10 | 18 | 1.00 | 1.03 | 36 | 0.44 | 0.69 | 25 | 1.52 | 1.42 | 30 | 0.73 | 0.74 |
| T11 | 19 | 0.89 | 0.88 | 39 | 0.41 | 0.59 | 25 | 1.40 | 1.50 | 30 | 0.70 | 0.75 |
| T12 | 18 | 0.72 | 0.75 | 38 | 0.32 | 0.47 | 25 | 1.08 | 1.32 | 30 | 0.60 | 0.67 |
| L1 | 16 | 0.94 | 0.93 | 39 | 0.51 | 0.56 | 27 | 1.59 | 1.12 | 30 | 1.20 | 1.16 |
| L2 | 17 | 0.94 | 0.97 | 40 | 0.93 | 0.80 | 26 | 2.04 | 1.11 | 30 | 1.47 | 1.17 |
| L3 | 16 | 1.19 | 1.11 | 39 | 1.23 | 1.13 | 27 | 2.11 | 1.09 | 30 | 1.47 | 1.07 |
| L4 | 17 | 1.24 | 1.03 | 40 | 1.45 | 1.13 | 27 | 2.19 | 1.00 | 29 | 1.69 | 1.07 |
| L5 | 16 | 1.38 | 1.09 | 40 | 1.28 | 1.15 | 27 | 1.78 | 1.05 | 29 | 1.83 | 1.14 |
| TOTAL | 407 | 0.80 | 0.92 | 896 | 0.55 | 0.79 | 588 | 1.20 | 1.23 | 687 | 0.74 | 0.95 |
| Male Adult Young (Individual N=9) | Female Adult Young (Individual N=41) | Male Adult Middle (Individual N=13) | Female Adult Middle (Individual N=30) | ||||||||||
| N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | ||
| C1 | L | 12 | 0.00 | 0.00 | 66 | 0.05 | 0.27 | 24 | 0.04 | 0.20 | 49 | 0.02 | 0.14 |
| R | 12 | 0.00 | 0.00 | 66 | 0.03 | 0.17 | 24 | 0.04 | 0.20 | 51 | 0.04 | 0.20 | |
| C2 | L | 16 | 0.13 | 0.50 | 64 | 0.08 | 0.32 | 25 | 0.40 | 1.00 | 57 | 0.14 | 0.40 |
| R | 16 | 0.06 | 0.25 | 64 | 0.08 | 0.32 | 25 | 0.60 | 1.19 | 55 | 0.16 | 0.42 | |
| C3 | L | 18 | 0.33 | 0.84 | 62 | 0.10 | 0.39 | 26 | 1.35 | 1.32 | 48 | 0.31 | 0.78 |
| R | 18 | 0.11 | 0.32 | 62 | 0.06 | 0.31 | 26 | 0.96 | 1.18 | 48 | 0.17 | 0.56 | |
| C4 | L | 18 | 0.44 | 0.86 | 64 | 0.08 | 0.41 | 24 | 0.92 | 1.14 | 44 | 0.34 | 0.91 |
| R | 18 | 0.44 | 0.86 | 64 | 0.03 | 0.18 | 24 | 0.88 | 0.99 | 44 | 0.20 | 0.59 | |
| C5 | L | 18 | 0.22 | 0.94 | 70 | 0.09 | 0.41 | 24 | 0.71 | 0.95 | 48 | 0.40 | 0.87 |
| R | 18 | 0.28 | 0.46 | 70 | 0.07 | 0.26 | 24 | 0.88 | 0.95 | 48 | 0.21 | 0.41 | |
| C6 | L | 18 | 0.33 | 0.97 | 70 | 0.06 | 0.23 | 22 | 0.59 | 0.96 | 47 | 0.26 | 0.61 |
| R | 18 | 0.22 | 0.43 | 70 | 0.04 | 0.20 | 22 | 0.41 | 0.67 | 47 | 0.19 | 0.54 | |
| C7 | L | 18 | 0.39 | 1.04 | 74 | 0.04 | 0.20 | 24 | 0.96 | 1.16 | 48 | 0.29 | 0.77 |
| R | 18 | 0.39 | 1.04 | 74 | 0.03 | 0.16 | 24 | 0.71 | 0.91 | 48 | 0.27 | 0.61 | |
| T1 | L | 16 | 0.44 | 1.03 | 76 | 0.05 | 0.22 | 26 | 0.42 | 0.90 | 54 | 0.20 | 0.56 |
| R | 16 | 0.50 | 1.10 | 76 | 0.07 | 0.25 | 26 | 0.46 | 0.81 | 54 | 0.17 | 0.47 | |
| T2 | L | 16 | 0.13 | 0.34 | 72 | 0.04 | 0.20 | 26 | 0.04 | 0.20 | 52 | 0.10 | 0.30 |
| R | 16 | 0.13 | 0.34 | 72 | 0.08 | 0.40 | 26 | 0.08 | 0.27 | 52 | 0.10 | 0.30 | |
| T3 | L | 14 | 0.36 | 0.63 | 78 | 0.04 | 0.19 | 26 | 0.04 | 0.20 | 48 | 0.02 | 0.14 |
| R | 14 | 0.50 | 0.94 | 78 | 0.08 | 0.39 | 26 | 0.08 | 0.39 | 48 | 0.02 | 0.14 | |
| T4 | L | 12 | 0.50 | 0.90 | 72 | 0.03 | 0.17 | 24 | 0.00 | 0.00 | 52 | 0.06 | 0.31 |
| R | 12 | 0.67 | 1.15 | 72 | 0.03 | 0.17 | 24 | 0.00 | 0.00 | 52 | 0.08 | 0.33 | |
| T5 | L | 14 | 0.21 | 0.58 | 74 | 0.08 | 0.27 | 22 | 0.00 | 0.00 | 48 | 0.08 | 0.28 |
| R | 14 | 0.21 | 0.58 | 74 | 0.08 | 0.27 | 22 | 0.00 | 0.00 | 48 | 0.17 | 0.48 | |
| T6 | L | 12 | 0.33 | 0.78 | 70 | 0.09 | 0.28 | 24 | 0.00 | 0.00 | 52 | 0.12 | 0.32 |
| R | 12 | 0.33 | 0.78 | 70 | 0.11 | 0.36 | 24 | 0.00 | 0.00 | 52 | 0.15 | 0.46 | |
| T7 | L | 12 | 0.42 | 0.79 | 70 | 0.06 | 0.23 | 22 | 0.00 | 0.00 | 50 | 0.12 | 0.33 |
| R | 12 | 0.42 | 0.79 | 70 | 0.10 | 0.42 | 22 | 0.00 | 0.00 | 50 | 0.16 | 0.42 | |
| T8 | L | 14 | 0.29 | 0.73 | 72 | 0.07 | 0.26 | 24 | 0.04 | 0.20 | 54 | 0.11 | 0.37 |
| R | 14 | 0.29 | 0.61 | 72 | 0.06 | 0.23 | 24 | 0.04 | 0.20 | 54 | 0.13 | 0.44 | |
| T9 | L | 14 | 0.57 | 1.16 | 68 | 0.15 | 0.36 | 24 | 0.13 | 0.34 | 54 | 0.31 | 0.58 |
| R | 14 | 0.57 | 1.16 | 68 | 0.16 | 0.37 | 24 | 0.17 | 0.48 | 54 | 0.28 | 0.60 | |
| T10 | L | 16 | 0.75 | 1.13 | 66 | 0.23 | 0.42 | 24 | 0.17 | 0.48 | 54 | 0.30 | 0.60 |
| R | 16 | 0.75 | 1.13 | 66 | 0.21 | 0.41 | 24 | 0.54 | 1.22 | 54 | 0.24 | 0.47 | |
| T11 | L | 16 | 0.38 | 0.62 | 70 | 0.26 | 0.56 | 26 | 0.38 | 1.13 | 53 | 0.38 | 0.77 |
| R | 16 | 0.38 | 0.62 | 70 | 0.24 | 0.55 | 26 | 0.38 | 1.13 | 53 | 0.38 | 0.74 | |
| T12 | L | 14 | 0.43 | 0.65 | 72 | 0.21 | 0.50 | 26 | 0.31 | 1.09 | 48 | 0.29 | 0.68 |
| R | 14 | 0.36 | 0.50 | 72 | 0.21 | 0.50 | 26 | 0.00 | 0.00 | 48 | 0.38 | 0.79 | |
| SUM | 576 | 0.35 | 0.79 | 2660 | 0.09 | 0.33 | 926 | 0.34 | 0.82 | 1920 | 0.19 | 0.53 | |

